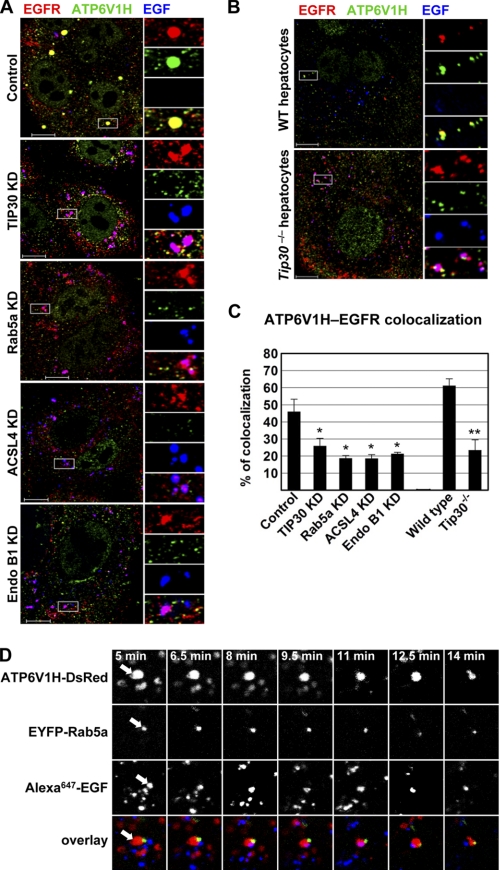
FIGURE 8.
Rab5a vesicles transport V-ATPases to early endosomes. A, depletion of TIP30, ACSL4, or Endo B1 inhibits the loading of V-ATPases on endocytic vesicles. Cells were immunostained for EGFR (red) and ATP6V1H (green) after 60 min of EGF (blue) internalization. Results are typical and representative of three experiments on cells from two different shRNAs. The boxed areas are magnified. Scale bars, 10 μm. KD, knockdown. B, deletion of Tip30 in mouse primary hepatocytes results in mislocalization of V-ATPases. Immunostaining was performed as described in A. Scale bars, 10 μm. C, colocalization between V-ATPases and EGFR was analyzed using MBF_ImageJ. Pearson's colocalization coefficients were calculated and converted to percentages. *, p < 0.05, **, p < 0.01, relative to control or wild type cells; Student's t test. D, Alexa647-EGF is released after EGF endocytic vesicles merge with Rab5a vesicles. Live cells expressing ATP6V1H-DsRed (red) and EYFP-Rab5a (green) were imaged by confocal microscopy at the indicated times after Alexa647-EGF internalization, and images of a single focal plane were acquired. A typical EGF endocytic vesicle movement was shown. Arrows point toward the two vesicles undergoing merge and EGF release.