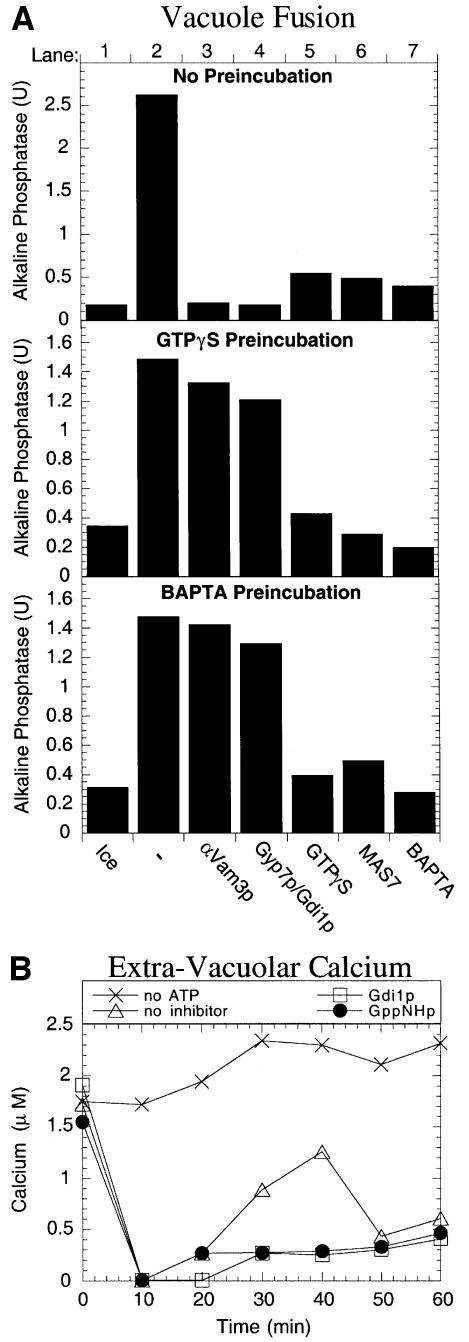
Fig. 8. The molecular requirements for the late-stage GTPase activity and Ca2+ are functionally linked. (A) Reactions blocked for fusion by either the calcium chelator BAPTA or GTPγS are still sensitive to the other inhibitor. Fusion reactions (120 µl) were incubated at 27°C for 45 min in the presence of 2 mM Mg-GTPγS (middle panel) or 2.5 mM BAPTA (bottom panel) to allow docking without fusion. One set of reactions was not pre-treated (top panel). Vacuoles were re-isolated and aliquots added to tubes containing reaction buffer on ice or reaction buffer with either no inhibitor, anti-Vam3p antibodies, Gdi1p + Gyp7p, 2 mM GTPγS, 50 µM MAS7 or 2.5 mM BAPTA. Reactions were supplemented with LMA1 and calmodulin (5 µg/ml and 150 µg/ml, respectively) and incubated (27°C, 90 min) before assaying for alkaline phosphatase activity. (B) Non-hydrolyzable GTP analogs and G-protein ligands inhibit the release of Ca2+ during vacuole fusion reactions. Fusion reactions (700 µl) were incubated at 27°C in the presence of Gdi1p, 2 mM Mg-GppNHp or no inhibitor. A control reaction did not contain an energy source. At the indicated times, 100 µl samples were removed, centrifuged at 9 000 g for 5 min, and 90 µl of the supernatant were assayed for Ca2+ (see Material and methods).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.