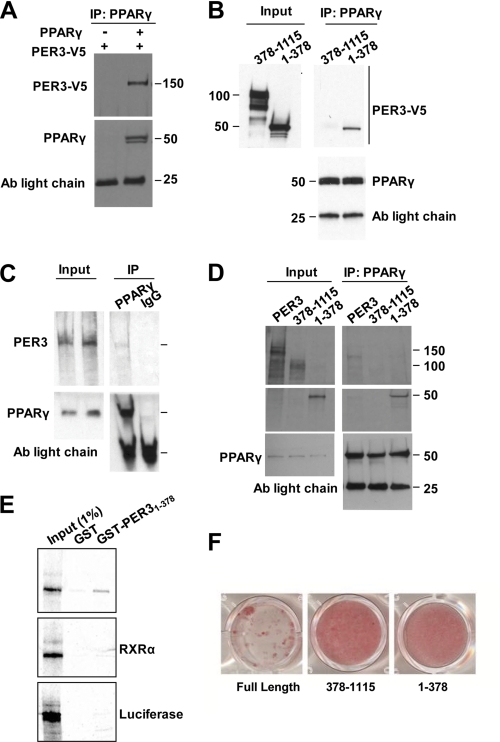
FIGURE 3.
Per3 interacts with PPARγ. A, immunoprecipitation with anti- PPARγ antibody (Ab) from COS-7 cells expressing exogenous PER3-V5 with and without PPARγ followed by Western blots with anti-V5 and anti-PPARγ is shown. B, immunoprecipitation from COS-7 cells co-expressing PPARγ and either the PER3 N-terminal fragment or PER3 with the N terminus deleted with anti-PPARγ antibody followed by Western blots using anti-V5 or anti-PPARγ. C, immunoprecipitation with anti-PPARγ or IgG control antibody of recombinant PPARγ and 35S-labeled recombinant PER3. Input lanes showing PER3 were used to verify that co-precipitated PER3 band is at the correct molecular size. Membranes were probed with anti-PPARγ to confirm the presence or absence of PPARγ. D, immunoprecipitation with anti-PPARγ antibody of recombinant PPARγ and [35S]Met-labeled recombinant full-length PER3 or PER3 deletion mutants. Membranes were probed with anti-PPARγ to confirm the presence or absence of PPARγ. Antibody light chain bands are shown as loading controls. E, a GST fusion with the N-terminal PER3 region was expressed in E. coli, and recombinant [35S]Met-labeled PPARγ was recovered from bacterial lysate in a GST pulldown assay. GST alone did not pull down PPARγ from the lysate. GST-PER3 did not pull down recombinant [35S]Met-labeled RXRα or luciferase. Input controls served as molecular size markers for the recombinant proteins. F, confluent 3T3-L1 cells expressing full-length or fragments of Per3 were induced to undergo adipogenesis with the glucocorticoid mixture and cultured for 6 days. After 6 days, cells were fixed and stained with Oil Red O to identify adipocytes.