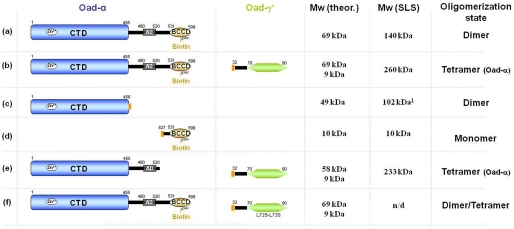
FIGURE 1.
Protein constructs used in this study. Different versions of Oad-α from V. cholerae (first column) were co-expressed and purified with or without the predicted soluble domain of Oad-γ (Oad-γ′; second column): a, full-length Oad-α; b, full-length Oad-α and Oad-γ′; c, N-terminal CT domain of Oad-α; d, C-terminal BCC domain of Oad-α together with Oad-γ′; e, BCC domain-depleted Oad-α mutant simultaneously with Oad-γ′; f, the same as b but with a Oad-γ′ double mutant in which Leu72-Leu73 residues were substituted with serines (L72S/L73S). The molecular mass was theoretically calculated for a single polypeptide chain from the amino acid sequence (Mw theor.) and experimentally analyzed by SEC-SLS experiments (Mw SLS), except for construct f, in which the molecular mass estimation was performed from HPLC experiments (Fig. 5). The last column summarizes the estimated oligomerization state in solution derived from this study. The orange rectangle represents a His tag. Note that comparable results were obtained in the absence of the His tag. Values were obtained from Ref. 9; n/d, not determined.