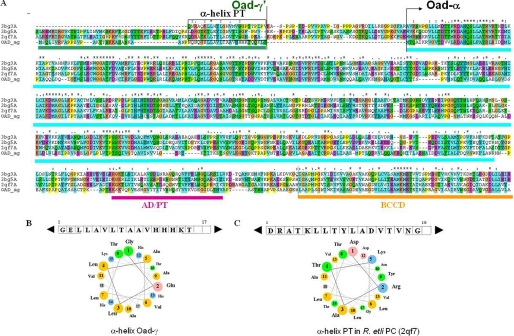
FIGURE 3.
Sequence comparison between Oad subunits and PC (without its BC domain). A, multiple sequence alignment between an Oad-α/γ′ chimera and different PC proteins of known structure represented by the codes in the Protein Data Bank: 3bg3, human PC; 3bg5, Staphylococcus aureus PC; 2qf7, R. etli PC; OAD_αγ, Oad-α, and the soluble domain of Oad-γ (Oad-γ′) sequence of V. cholerae oxaloacetate decarboxylase 2. The amino acid sequence of the γ subunit of OAD is marked in green; the CT domain, the association domain/tetramerization domain (AD/TD), and the BCC domain of Oad-α are marked in cyan, purple, and orange, respectively. B, α-helix in PC equivalent to Oad-γ that is involved in PT stabilization is depicted in a rectangle. C, properties of the α-helix in Oad-γ′ related to an equivalent α-helix in PT domain of R. etli are PC illustrated by the amino acid sequence and helical wheel presentation.