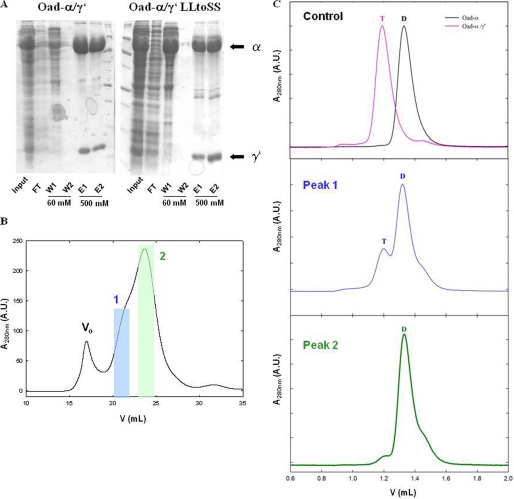
FIGURE 5.
Disruption of Oad-α and Oad-γ′ interaction by point mutations in Oad-γ′. A, SDS-PAGE protein separation of Oad-α/γ′and Oad-α/γ′L72S/L73S complexes (constructs b and f, respectively, Fig. 1) during protein affinity purification. Input, flow-through (FT), first (W1) and last (W2) washes with 60 mm imidazole and elution with 500 mm imidazole (E1 and E2) are shown. B, quaternary structure analysis of the mutant complex by SEC. Two peaks containing Oad species are clearly distinguished (blue and green, respectively). C: first panel on the right, control experiment in which the elution volume of Oad-α and Oad-α/γ′ allows distinguishing between dimer (D) and tetramer (T) species. Second and third panels on the right, second run over a SEC column of peaks 1 (blue) and 2 (green), respectively. Dimer or tetramer species are indicated with a D and T, respectively. Note that Oad-γ′ does not absorb at 280 nm due to the absence of aromatic amino acids in its sequence.