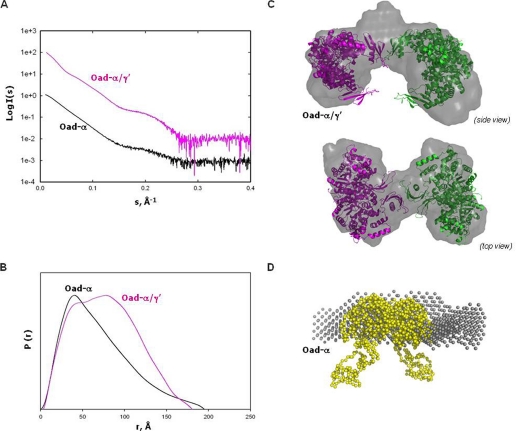
FIGURE 6.
SAXS analysis of Oad-α/γ′ and Oad-α. A, extrapolation to zero concentration of the SAXS experimental scattering profiles. B, pair-distribution functions of the curves in A. C, rigid-body modeling and ab initio shape reconstruction (gray) of Oad-α/γ′. Two Oad-α dimers (pink and green, respectively) are fitted into the ab initio models. The two dimers interact by two association domains coupled with two Oad-γ′ subunits. D, overlapping of a Oad-α dimer model (yellow) constructed by fusion of the 2nx9 and PT-BCC domain from 2qf7 with the low resolution envelope obtained from ab initio shape reconstitution (gray) from SAXS-derived data of purified Oad-α protein.