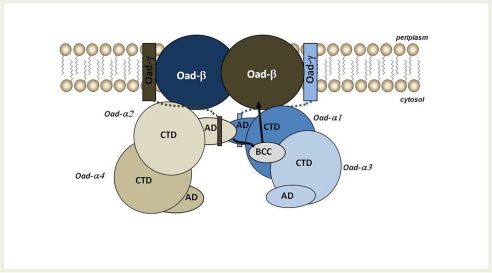
FIGURE 8.
Working model of OAD complex organization in the membrane. According to this model, two Oad-α dimers form a functional unit upon interaction with Oad-γ at the membrane. The soluble α-helices of two intrinsic Oad-γ subunits (shown as rectangles connected to the membrane by dotted lines) would promote the interaction between AD of two Oad-α monomers of opposite dimers. As a consequence, the BCC domain of one AD-mediated interacting Oad-α subunit would be located near the CT domain of an opposite monomer. The most likely mechanism supports a conformational flexibility of the BCC domain and would involve the carboxyl transfer to biotin in the BCC domain from the CT domain of an opposite monomer. Upon a conformational reorganization, the BCC domain would move toward one Oad-β subunit at the membrane, favoring its decarboxylation that results in the translocation of sodium ions across the bacterial membrane.