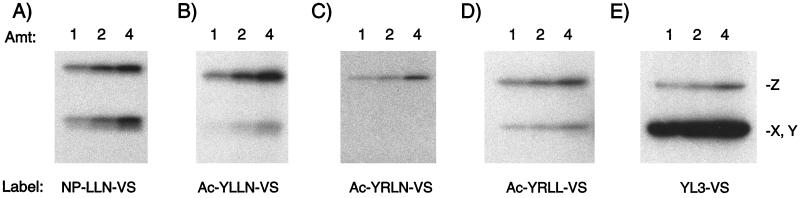
Figure 5.
Tuning the selectivity of affinity labels for the proteasome's multiple active sites. Direct labeling of proteasomes in crude NIH 3T3 lystates using a series of peptide vinyl sulfones. Beginning on the right (A) with the broadly reactive compound NP-LLN-VS and replacing the nitrophenol cap with tyrosine residue (Ac-YLLN-VS; B) increases labeling of the Z subunit. Changing the P3 leucine residue to arginine (Ac-YRLN-VS; C) yields a Z-subunit selective inhibitor. Changing the P1 to leucine (Ac-YRLL-VS; D) restores modest labeling of the X and Y subunits. Finally, returning the P3 residue to leucine (YLLL-VS; E) results in predominant labeling of the X and Y subunits of the proteasome.