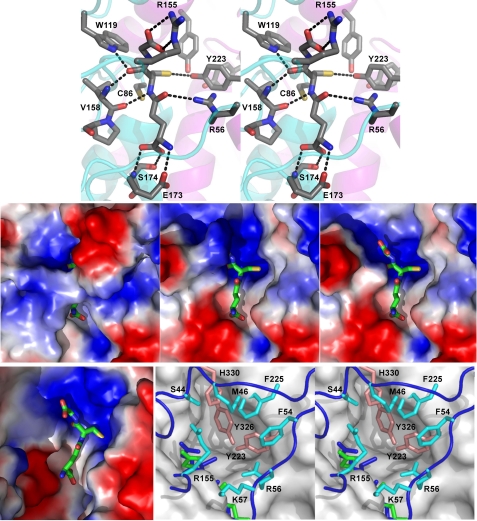
FIGURE 5.
Substrate-binding sites of PcGSTO1 and HsGSTO1-1 (10). Top, stereo view of the hydrogen bond network formed by the active site residues around GSH in PcGSTO1. The schematic trace of the polypeptide main chain is suggested in cyan (thioredoxin-like domain) and purple (α-helix domain). Middle, PcGSTO1 electrostatic surface (blue, positive charge; red, negative charge) of PcGSTO1 in the region of GSH (shown as sticks). Left panel, calculated with all the residues observed for monomer A in the crystallographic model (Glu38–Asp351); middle panel, calculated without the N-terminal coil (i.e. with residues Tyr78–Asp351); right panel, same as previous but without Arg155. Bottom, HsGSTO1-1 active site compared with PcGSTO1. Left panel, electrostatic surface calculated for the HsGSTO1-1dimer, in the region of GSH (shown as sticks). Right panel, stereo view of the accessible surface of HsGSTO1-1 (gray) in the region of GSH (green), superimposed to the PcGSTO1 active site containing the GSH (blue sticks). Residues Tyr223, Tyr326, and His330 fill the pocket defined by Board et al. (10) as the HsGSTO1-1 active site. Residues in cyan and in orange form a second and a third layers of residues that almost close the PcGSTO1 active site.