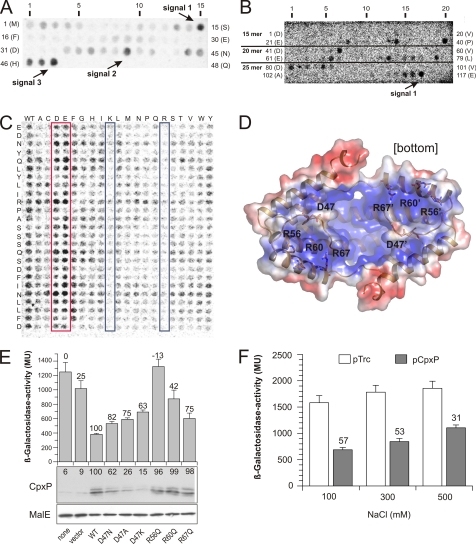
FIGURE 5.
Protein-protein interaction between CpxP and CpxA. A, a peptide array derived from the sequence of CpxP was screened for binding of the 35S-labeled CpxA sensor domain (CpxA28–164-His6) as described under “Experimental Procedures.” Rows are labeled for peptide numbers and the N-terminal peptide residues. Those spots exhibiting the strongest signal within a serial of increasing signals are highlighted. B, reverse experiment to A. Peptide arrays (15-mer, 20-mer, and 25-mer) derived from the sequence of the CpxA sensor domain were prepared and incubated with 35S-labeled CpxP-His6. One spot exhibiting the strongest signal within a serial of increasing signals of the 25-mer peptide array that covers sequences of signals of the 15-mer and 20-mer peptide arrays is highlighted. C, complete substitutional and length analysis of the interacting 25-mer peptide of the CpxA-derived peptide recognized by CpxP was generated using the software LISA and subsequently synthesized as described under “Experimental Procedures.” Each amino acid of the peptides (25-mer) corresponding to signal 1 in B is substituted by all other 20 l-amino acids in alphabetical order (shown on top of the membrane) and tested for binding of 35S-labeled CpxP-His6. All spots in the left column comprise the wild-type sequence (WT) of the peptide. Those spots with increased negative or positive charged amino acids are highlighted by red and blue boxes, respectively. D, surface representation of CpxPΔ151 dimer. The electrostatic surface potentials were calculated using the program APBS (39) with the non-linear Poisson-Boltzmann equation and contoured at ± 3 k * T/e, where k is Boltzmann's constant; T, the temperature in K, and e, the charge of an electron. Negatively and positively charged surface areas are colored in red and blue, respectively. E, CpxP-mediated Cpx pathway inhibition was investigated as described for Fig. 3. F, the salt dependence of CpxP-mediated Cpx pathway inhibition was investigated by β-galactosidase activity measurement using the strain SP594 (16). CpxP was expressed from vector pTrc99A (Invitrogen). Averages ± S.E. from five independent determinations each with three replicates are shown. Numbers above the bars give the relative inhibition of β-galactosidase activity at the indicated NaCl concentrations.