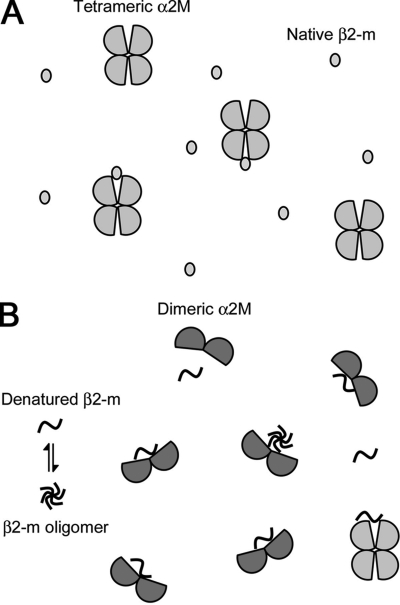
FIGURE 8.
Schematic model of the denaturation-driven interaction of α2M with β2-m. A, under normal conditions, tetrameric native α2M may interact weakly with native β2-m. B, under amyloidogenic denaturing conditions (e.g. SDS, lipids, low pH), tetrameric α2M is partly converted to dimeric form. β2-m is also partially unfolded, and this β2-m conformer may weakly and reversibly aggregate into small oligomers. Because the surface hydrophobicity of dimeric α2M is clearly higher than that of tetrameric α2M, dimeric α2M can strongly interact with denatured monomeric/oligomeric β2-m with exposed hydrophobic surfaces. Tetrameric α2M may also interact with denatured monomeric/oligomeric β2-m. By binding to the denatured monomeric/oligomeric β2-m, α2M may inhibit the formation of β2-m amyloid fibrils.