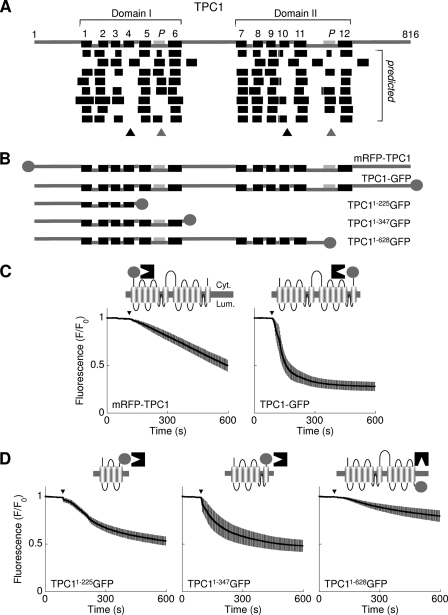
FIGURE 1.
Domain architecture of TPC1. A, top, schematic depiction of human TPC1 based on alignment to voltage-sensitive Ca2+ and Na+ channels showing the positions of the predicted TM regions (numbered, black) and pores (P, pale gray). Two repeated domains (I and II) are identified. Linker regions predicted to be cytosolic (upper connectors) or luminal (lower connectors) are shown. Bottom, unbiased predictions of the TM regions derived using (from top to bottom) Das, SOSUI, TMHMM, HMMTOP, TMPred, Split Server, TMMOD, and Phobius (see supplemental Table S1). Ambiguous predictions of TM4/10 and the pores are highlighted by the black and gray triangles, respectively. B, schematic depiction of the human TPC1 fusion proteins used, where circles represent the fluorescent tags. C and D, fluorescence protease protection assays using cells expressing the indicated TPC1 fusion protein. Trypsin was added at the arrowhead. Fluorescence signals are normalized to initial fluorescence and are presented as means ± S.E. from >40 cells derived from at least 4 fields from two independent transfections. Cartoons show a schematic of the channel with the assumed position of the tag (circle), which faces either the cytoplasm (Cyt.), where it is accessible to trypsin (black structure); or the lumen (Lum.), where it is protected.