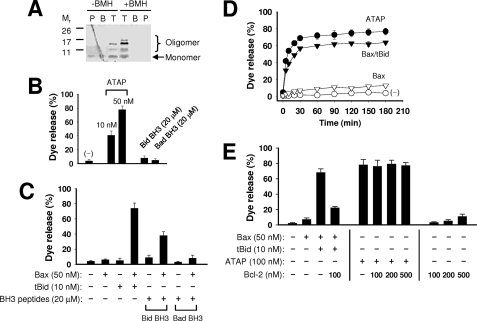
FIGURE 5.
ATAP forms large pores in the MOM-like liposomal membrane. A, oligomerization of ATAP in the liposomal membrane was examined by BMH cross-linking. The samples were fractionated using the step sucrose gradient centrifugation. The resulting top (T), bottom (B), and pellet (P) fractions contained liposome-bound, -soluble, and -aggregated peptide and adducts, respectively. All fractions were subjected to Tris-Tricine SDS-PAGE, and the peptide and adducts were visualized by silver staining. B, release of 10-kDa CB dextrans from the MOM-liposome by the indicated concentrations of ATAP peptide or BH3 peptides was monitored using the fluorescence quenching method. Data shown are the average extent of release after 5 h of incubation from three independent experiments with standard deviation. C, release of 10-kDa CB dextrans from the MOM-liposome by BH3 peptides (20 μm) in the presence of Bax (50 nm) was monitored as above. Data shown are averages from three independent experiments with standard deviation. D, release kinetics of 10-kDa CB dextrans by ATAP (50 nm) or Bax (50 nm) together with or without tBid (10 nm) was monitored. A negative control shows an effect by DMSO vehicle without any peptide. Data shown are averages from three independent experiments with standard deviation. E, release of 10-kDa CB dextrans from 12.5 μm Ni2+-chelating liposome by ATAP peptide or tBid-activated Bax in the presence or absence of His6-Bcl-2ΔTM was monitored as above. Data shown are averages from three independent experiments with standard deviation. The concentration of ATAP was doubled compared with that in B–D because the batch of peptide used here is less active than the one used in B–D.