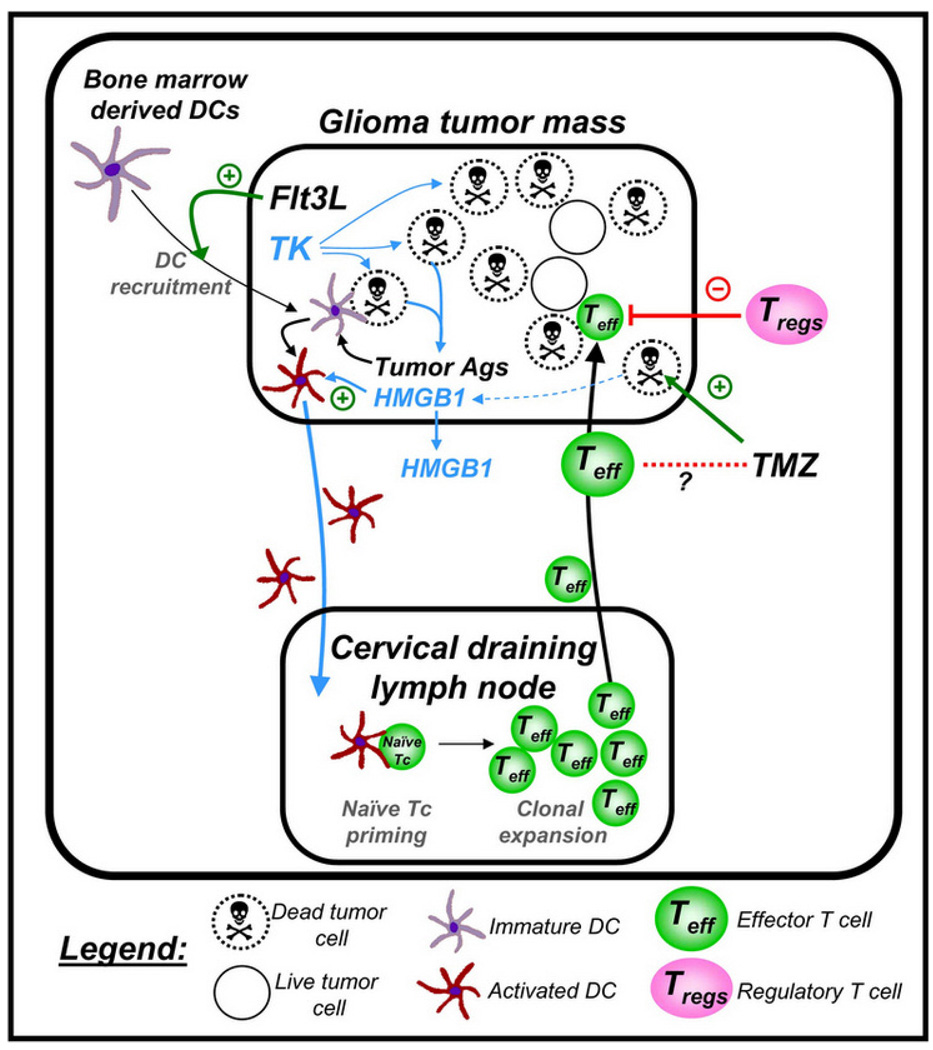
Figure 1.
Schematic depiction of the mechanism underlying the induction of a specific anti-GBM immune response using Flt3L/TK gene therapy. First generation adenoviral vectors encoding Flt3L and HSV1-TK are injected into an intracranial brain tumor; ganciclovir is administered systemically. Expression of HSV1-TK in the presence of ganciclovir mediates tumor cell death, releasing endogenous brain tumor antigens and danger signals, including HMGB1. Intratumoral expression of Flt3L recruits dendritic cells (DCs) into the brain tumor milieu where they take up brain tumor antigens released from the dying GBM cells and present them on their MHC complexes. This phenomenon is dependent on the TLR2/RAGE agonist HMGB1, which is released from dying tumor cells. The DCs loaded with brain tumor antigens migrate to the cervical draining lymph nodes where there present brain tumor antigens to naïve T cells, mediating a clonal expansion of brain tumor specific anti-GBM effector T cells. The GBM specific effector T cells then migrate back into the brain and kill residual GBM cells. Therapeutic efficacy of the approach is not diminished when mice are also treated with the chemotherapy temozolomide (TMZ).