Abstract
The epileptic hippocampus has an enhanced propensity for seizure generation, but how spontaneous seizures start is poorly understood. Using whole cell and field-potential recordings, this study explored whether repetitive perforant-path stimulation at physiological frequencies could induce epileptiform bursts in dentate gyrus minislices from rats with kainate-induced epilepsy. Control slices from saline-treated rats responded to single perforant-path stimulation with an excitatory postsynaptic potential (EPSP) and a single population spike in normal medium, and repetitive stimulation at different frequencies (0.1, 1, 2, 5, 10 Hz) did not cause significant increases in the responses. Most minislices (82%) from rats with kainate-induced epilepsy also responded to single perforant-path stimulation with an EPSP and a single population spike/action potential, but some slices (18%) had a more robust response with a prolonged duration and negative DC shift or responses with two to three population spikes. Repetitive perforant-path stimulation at 5–10 Hz, however, transformed the single-spike responses into epileptiform bursts with multiple spikes in half (52%) of the slices, while lower frequency (e.g., ≤1 Hz) stimulation failed to produce these changes. The emergence of epileptiform bursts was consistently associated with a negative field-potential DC shift and membrane depolarization. The results suggest that compared with the controls, the “gate” function of the dentate gyrus is compromised in rats with kainate-induced epilepsy, and epileptiform bursts (but not full-length seizure events) can be induced in minislices by repetitive synaptic stimulation at physiological frequencies in the range of hippocampal theta rhythm (i.e., 5–10 Hz).
INTRODUCTION
Epilepsy is characterized by spontaneous, recurrent seizures. Several pathological changes have been reported to occur in the dentate gyrus during epileptogenesis and to contribute to an enhanced propensity for seizure generation. These changes include loss of inhibitory interneurons (Buckmaster and Dudek 1997; Kobayashi and Buckmaster 2003; Obenaus et al. 1993) and sprouting of excitatory axons (Molnar and Nadler 1999; Tauck and Nadler 1985; Wuarin and Dudek 2001). Similar pathological changes may occur in other regions of the brain. Although these changes in the epileptic brain would favor the generation of seizures, spontaneous seizures in the epileptic brain only occur intermittently, and the onset of seizures is largely unpredictable and poorly understood.
In in vitro experiments, epileptiform bursts rarely occur in the dentate gyrus in slices prepared from epileptic animals unless the experimental conditions have been artificially manipulated to alter seizure threshold, such as blocking inhibition and/or increasing [K+]o (Hardison et al. 2000; Patrylo and Dudek 1998; Wuarin and Dudek 1996). The present study aimed to explore the question of how seizures start and propagate in a “natural” milieu (i.e., without blocking inhibition and manipulation of extracellular ion concentration) in the dentate gyrus. The dentate gyrus is traditionally considered as a “gate,” limiting activity entering hippocampus from entorhinal cortex in normal animals. However, earlier studies have shown that the gate function can be compromised by repetitive stimulation (i.e., so-called “maximal dentate activation”) (Stringer and Lothman 1989; Stringer et al. 1989). During epileptogenesis, the dentate gyrus undergoes multiple and consistent pathological changes that favor seizure generation (Dudek et al. 2002). Electrographic seizures in the dentate gyrus of chronically epileptic animals are preceded by increased neuronal firing (Bower and Buckmaster 2008) or associated with rhythmic electrographic spikes at 5–12 Hz (J. L. Hellier and F. E. Dudek, unpublished data). Therefore we hypothesized that stimulation patterns roughly comparable to normal physiological activity in slices from epileptic brain might cause activity-dependent changes that would induce epileptiform bursts. Using minislices of dentate gyrus (i.e., isolated from adjacent tissues, Fig. 1A) from rats with kainate-induced epilepsy, we tested whether repetitive synaptic activation of the granule cells could trigger epileptiform bursts in this region. Our results suggest that the gate function of the dentate gyrus is compromised, and repetitive perforant-path stimulation at physiological frequencies in the range of hippocampal theta rhythm (i.e., 5–10 Hz) can induce epileptiform bursts (but not full-length seizure events) in minislices of dentate gyrus from rats with kainate-induce epilepsy, compared with saline-treated control animals.
Fig. 1.
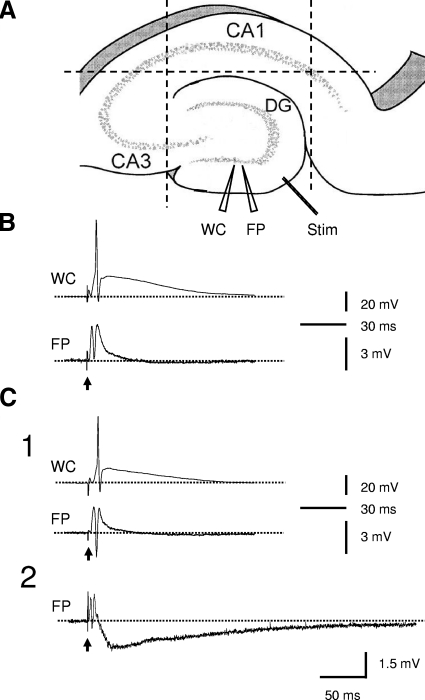
Protocol of the experiments and profile of the responses to individual perforant path stimulations in slices from control and kainate-treated rats. A: diagram showing experimental protocol. - - -, knife cuts to isolate dentate gyrus from CA3, CA1, and entorhinal cortex. WC, whole cell recording electrode; FP, field-potential recording electrode; Stim, stimulation electrode. B: typically, a dentate gyrus minislice from control rats responded to single perforant-path stimulation with an excitatory postsynaptic potential (EPSP) and a single population spike in field-potential recording and an action potential in whole cell recording. C: in slices from kainate-treated rats, the responses to single perforant path stimulations were more variable and robust. While most minislices showed responses similar to that in the controls (C1), some of the slices displayed responses with a prolonged negative field-potential shift and with or without preceding population spikes (C2). ↑, stimulations; - - -, the baseline potential levels.
METHODS
Animal model of epilepsy
To induce chronic epilepsy in rats, a repeated low-dose kainate-treatment protocol similar to previous studies (Hellier et al. 1998; Shao and Dudek 2004) was used. Briefly, male Sprague-Dawley rats (Harlan) weighing ∼175 g were injected hourly with kainate (5 mg/kg ip). Motor seizures normally occurred after one to three injections. Animals had recurring class IV/V seizures (Ben-Ari 1985; Racine 1972) for ≥3 h. Control rats received saline injections in parallel with kainate-treated rats. After kainate treatment, rats were monitored for 1–2 h/day, 3–5 day/wk (i.e., 6 h/wk) to determine whether they developed spontaneous convulsive seizures. All procedures with animals used in this study were approved by the Colorado State University and University of Utah Animal Care and Use Committees.
Brain slice preparation
Rats were used ≥3 mo after kainate or saline treatment when kainate-treated rats had developed spontaneous seizures. Rats were anesthetized with halothane and decapitated with a guillotine. The brains were quickly dissected out and placed in ice-cold oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM) 124 NaCl, 3 KCl, 26 NaHCO3, 1.4 NaH2PO4, 1.3 CaCl2, 1.3 MgSO4, and 11 glucose. Horizontal hippocampal slices of 450-μm thickness were cut parallel to the base of the brain with a vibroslicer (Campden Instrument, Lafayette, IN). Slices were trimmed, and three additional knife cuts were made to isolate the dentate gyrus from entorinal cortex, CA3 and CA1 (i.e., minislices, Fig. 1). Minislices were then transferred to and maintained in a storage chamber filled with ACSF (32–34°C) and continuously bubbled with 95% O2-5% CO2. Electrophysiological recordings were normally started 2 h after slice preparation.
Recording procedures and data acquisition
Dentate gyrus minislices were mounted onto a heated ramp-type interface chamber (32–34°C) for electrophysiological recording and were continuously perfused with gassed ACSF. Simultaneous extracellular field-potential and whole cell recordings (or extracellular field-potential recording alone) were performed with an Axoprobe-1A amplifier (for field-potential recording, Molecular Device, Foster City, CA) and an Axopatch-1D amplifier (for whole cell recording, Molecular Device). The recording electrodes were made from thick-wall borosilicate glass capillaries (1.65 mm OD, 1.2 mm ID, Garner Glass, Claremont, CA) with a P-87 Flaming-Brown puller (Sutter Instruments, Novato, CA) and placed in the granule cell layer in either blade of the dentate gyrus (Fig. 1A). A bipolar stimulating electrode composed of two Teflon-coated platinum wires [bare diameter: 25 μm, with coating: 75 μm; separation between wires: ∼50 μm (i.e., twice the thickness of coating)] was positioned in the lateral perforant path of the dentate gyrus to deliver synaptic stimulation (Fig. 1A). The stimulus intensity was adjusted to evoke a population spike in field-potential recordings (100–600 μA, 200 μs). For extracellular field-potential recordings, pipettes were filled with ACSF. For whole cell current-clamp recordings, pipettes were filled with intracellular solution containing (in mM) 120 K-gluconate, 1 NaCl, 5 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, and 2 ATP. The pH was adjusted to 7.2 with 5 M KOH. The calculated liquid junction potential between the intracellular solution and perfusing solution (ACSF) was ∼13 mV and was not corrected in the values reported here. Extracellular recordings were DC-coupled. All signals were sampled at 10 kHz, low-pass filtered at 2 kHz, and recorded on-line with pClamp 8.0 software (Clampex, Molecular Device) through a Digidata-1320A digitizer (Clampex, Molecular Device). Data were analyzed off-line with pClamp 8.0 (Clampfit, Molecular Device). The χ2 test was employed to compare the ratios between groups. The Student's t-test was used for comparisons between two groups. Data are expressed as means ± SE, and α = 0.05 in all tests.
RESULTS
Responses of dentate gyrus minislices to single perforant-path stimulation
In normal ACSF, individual synaptic stimulation at the lateral perforant path evoked an EPSP. An increase in stimulus intensity increased the amplitude of the EPSP and generated a population spike/action potential (Fig. 1B). A further increase in stimulus intensity increased the amplitude but not the number of population spikes. The duration of the response was usually <0.1s. This pattern of response (i.e., EPSP with single population spike/action potential and of short duration) was consistent in slices from control rats in response to perforant-path stimulation. The majority of the minislices (36 of 44 slices, 82%) from kainate-treated rats also responded to single perforant-path stimulation with responses similar to that in the controls (i.e., EPSP and a single population spike/action potential; Fig. 1C1). However, some slices (8 of 44 slices, 18%) from epileptic rats expressed more robust responses to single perforant-path stimulation. These responses include an initial EPSP followed by a clear negative DC shift lasting for ≤1–2 s (5 of 44 slices, 11%, Fig. 1C2) or responses with two or three population spikes (3 of 44, 7%, not shown). These prolonged responses in slices from rats with kainate-induced epilepsy are consistent with previous observations (Patrylo and Dudek 1999) and support the hypothesis that the dentate gyrus undergoes significant pathological changes toward an increased propensity for seizure generation during epileptogenesis.
Induction of epileptiform bursts by repetitive perforant-path stimulation
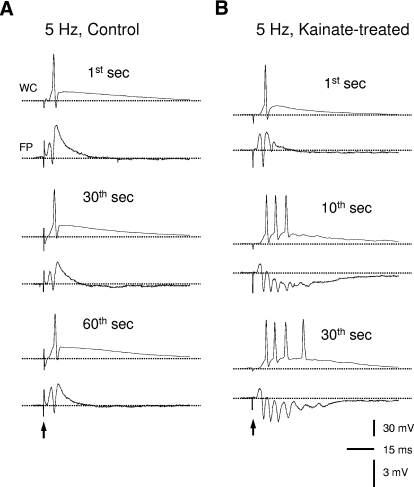
Next we tested the hypothesis that repeated physiological activity (i.e., comparatively low-frequency repetitive perforant-path stimulation) might cause epileptiform bursts in minislices from epileptic but not control rats. The stimulus intensity was adjusted to evoke a population spike of ∼2–4 mV and was similar for the control and epileptic groups (231 ± 18 μA, n = 36 slices vs. 250 ± 17 μA, n = 44 slices; P > 0.05, Student's t-test). Some dentate minislices appeared healthy (i.e., with large fEPSPs), but it was difficult to generate a population spike of 2–4 mV; these slices were also included in the study. In minislices from control rats, individual synaptic stimulations of the lateral perforant path typically elicited an EPSP with a single population spike/action potential (Figs. 2A and 1B). Repetitive stimulations (0.1–10 Hz, for ≤2 min) sometimes increased the amplitude of the population spike but did not develop multi-spike epileptiform bursts (Fig. 2A, n = 35 of 36 slices from 16 rats) except for one slice that developed multiple-spike burst at 5 Hz (1 of 36 slices, 2.8%; 1 in 16 rats, 6.3%). In addition, some of these slices (n = 18) were further tested with two- to fourfold greater stimulus intensities (≤600 μA) at 5 Hz, but none of them developed epileptiform bursts (data not shown). Thus the dentate gyrus gate in the control rats seemed mostly intact under the current experimental conditions. In contrast, half of the slices from rats with kainate-induced epilepsy (23 of 44 slices, 52%, P < 0.001; 15 of 24 rats, 63%, P < 0.001, χ2 test) developed multispike epileptiform bursts during perforant-path repetitive stimulation at 5 Hz. The initial responses with a single spike/action potential (Fig. 2B, top) were transformed into epileptiform bursts with multiple population spike/action potentials (Fig. 2B, middle and bottom). The transition from a single-spike response to a multispike burst took a variable period of time ranging from a few seconds to a few tens of seconds and was closely associated with the emergence of a negative shift of the field-potential baseline (see following text in Fig. 4). Sixteen of the 21 slices that showed no increase in responses during 5-Hz repetitive stimulation were further tested with greater stimulus intensities. As a result, 5 of them initially showed no change during repetitive stimulation but then developed epileptiform bursts with twofold greater stimulus intensity (320 ± 49 pA, from 160 ± 25 pA, n = 5), whereas the rest of the 11 slices remained unchanged with a similar increase in stimulus intensity. In those slices from epileptic rats that responded to individual perforant-path stimulation with a prolonged response, repetitive stimulation further intensified the responses and produced multispike bursts (data not shown). These data suggest that the dentate gate is significantly comprised in slices from rats with kainate-induced epilepsy, such that epileptiform bursts can be induced by relatively physiological activity in normal medium.
Fig. 2.
Perforant-path repetitive stimulation at 5 Hz induced epileptiform bursts in slices from rats with kainate-induced epilepsy but not control rats. A: simultaneous whole cell (WC, top) and field-potential (FP, bottom) recordings showing representative responses to perforant-path stimulations in slices from control rats. The initial response had a single population spike/action potential (i.e., at 1st second) and did not increase during repetitive stimulation (e.g., at 30th and 60th seconds). B: by contrast, 5-Hz repetitive stimulation transformed the initial responses with a single population spike/action potential into epileptiform bursts with multiple population spikes/action potentials in slices from rats with kainate-induced epilepsy. ↑, stimulations; - - -, the baseline potential levels.
Fig. 4.
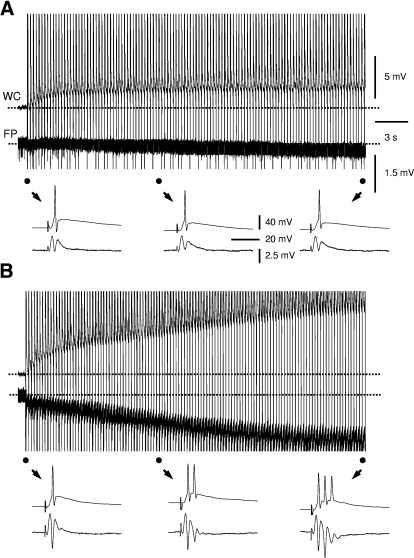
Repetitive stimulation-induced epileptiform bursts were associated with a negative DC shift in field-potential and depolarization. A and B: simultaneous whole cell (WC, top) and field-potential (FP, bottom) recordings showing changes in membrane potential and the baseline field-potential during 5-Hz perforant-path repetitive stimulation. In slices from rats with kainate-induced epilepsy, repetitive stimulation caused a DC shift in field potential (B, bottom), which was absent or negligible in slices from control rats (A, bottom). The initial, middle, and later responses (●) to repetitive stimulations in both groups were shown in expanded scale below as indicated ↓. Repetitive stimulation caused a modest depolarization in dentate granule cells in slices from control rats (A, top) and appeared to cause a larger depolarization in granule cells in slices from rats with kainate-induced epilepsy (B, top). - - -, the baseline voltage levels. Scale bars in A apply to B.
Frequency dependence of repetitive stimulation to induce epileptiform bursts
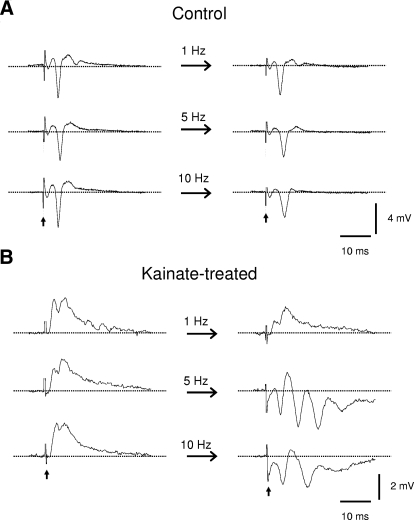
To determine the most effective frequency to induce the epileptiform bursts, different stimulation frequencies (0.1, 0.5, 1, 2–3, 5. and 10 Hz) were tested. In all but one slice (35 of 36, 97.2%) from control rats, repetitive stimulations at any of the tested frequencies failed to transform a single-spike response into a multiple-spike epileptiform burst (Fig. 3A). In minislices from epileptic rats, only 3 of 32 slices (9.4%) developed epileptiform bursts during repetitive perforant-path stimulation at 1–3 Hz (data not shown). The same slices developed multispike, epileptiform bursts when the frequency of repetitive stimulation was increased to 5 Hz (Fig. 3B). A further increase in the frequency to 10 Hz also induced epileptiform bursts (Fig. 3B). However, the responses tended to decrease or even collapse as 10-Hz stimulation persisted (data not shown). Thus repetitive stimulation at physiological frequencies in the range of hippocampal theta rhythm (i.e., 5–10 Hz) effectively induced epileptiform bursts in a fraction (∼50%) of slices from rats with kainate-induced epilepsy (Fig. 3B).
Fig. 3.
The most effective frequency to induce epileptiform bursts appeared to be 5 Hz. A: examples showing responses in slices from control rats to repetitive stimulations at low (i.e., 1 Hz), medium (5 Hz), and high (10 Hz) frequency. Repetitive stimulation failed to induce epileptiform bursts in nearly all slices from control rats regardless of the frequency of stimulations. B: similar stimulation protocol as in the preceding text, but in slices from rats with kainate-induced epilepsy. Perforant-path repetitive stimulation-induced epileptiform bursts occurred at a range of frequency from 1 to 10 Hz but most often occurred at 5 Hz.
Repetitive stimulation induced a significant negative DC shift in field potential and membrane depolarization
Interestingly, the occurrence of multispike epileptiform bursts during repetitive stimulation was consistently accompanied by a negative DC shift in the field-potential (1.4 ± 0.1 mV, n = 23 slices; Fig. 4B), a phenomenon similar to that seen during “maximal dentate activation” in vivo (Stringer and Lothman 1989; Stringer et al. 1989). In slices from control rats (and those slices from epileptic rats that failed to induce epileptiform bursts), a negative shift of the field potential was absent or negligible during repetitive stimulation (0.16 ± 0.03 mV, n = 54 slices, P < 0.001; Fig. 4A). Thus the negative DC shift in field potential is closely related to and may be important for the development of epileptiform bursts by repetitive stimulation. In addition, using simultaneous whole cell recording, we observed membrane potential changes of the dentate granule cells during the DC shift in the field potential. Repetitive stimulation caused a modest depolarization in granule cells (Fig. 4A, top) or hyperpolarization (not shown) in slices from control rats (average membrane potential change: 1.3 ± 1.6 mV, n = 5 cells from 5 slices). In slices from rats with kainate-induced epilepsy, repetitive stimulation caused a larger depolarization in dentate granule cells (8.6 ± 1.6 mV, P < 0.01, n = 6 cells from 6 slices, Fig. 4B, top).
DISCUSSION
The main findings in the present study were that in normal medium, repetitive synaptic stimulation at physiological frequencies in the range of hippocampal theta rhythm (i.e., 5–10 Hz) induced epileptiform bursts in a fraction (∼50%) of the dentate gyrus minislices from rats with kainate-induced epilepsy but not controls. The induction of epileptiform bursts was closely associated with a negative DC shift in the field potential and membrane depolarization.
Activity-induced epileptiform bursts and impaired dentate gate function in rats with kainate-induce epilepsy
Earlier in vitro studies have shown that the dentate gyrus has an enhanced seizure propensity in slices from the epileptic brain, which can be “unmasked” by blocking GABAergic inhibition and/or elevating extracellular potassium (Hardison et al. 2000; Patrylo and Dudek 1998; Wuarin and Dudek 1996). However, whether or how these conditions may actually occur in epileptic patients or animal models in vivo is unknown. Earlier in vivo studies in naïve animals suggest that the dentate gyrus can be maximally activated after trains of high-frequency (10–30 Hz) repetitive stimulation (Stringer and Lothman 1989; Stringer et al. 1989). Therefore it is possible that in the epileptic brain, which has undergone many pathological changes, repetitive physiological stimuli (which may represent normal signaling in the naïve brain), may cause exaggerated responses in the epileptic brain and then lead to the emergence of epileptiform bursts. The finding that a single episode of repetitive stimulation at physiological frequencies in the range of hippocampal theta rhythm (i.e., 5–10 Hz), compared with multiple episodes of 10–30 Hz stimulation for the maximal dentate gyrus activation in normal animals (Stringer and Lothman 1989; Stringer et al. 1989), effectively induced multispike bursts implies that the “gate-keeper” function of the dentate gyrus is impaired in epileptic animals. Although our data support the hypothesis that the dentate gate function is compromised in rats with kainate-induced epilepsy, about half (48%) of the dentate minislices from epileptic rats did not produce epileptiform bursts during repetitive stimulation. This might reflect a variable degree of pathological changes across the dentate gyrus. Another possibility is that the abnormal responses observed in the present study are not present in all slices from each animal because some of them have better preserved local circuitry (depending on the angle of the slice). These variables may also explain the observation that many minislices showing or not showing bursting were from the same epileptic animals. This made the correlation of the failure of gate function in minislices with the severity of epilepsy in the intact animals difficult. Also, because synaptic reorganization almost certainly occurs in many temporal and limbic structures in addition to the dentate gyrus, and seizure onsets are variable across brain structures (Bertram 2009), it seems quite possible that the changes reported here could be obscured by other alterations in the brain of kainate-treated rats. It may also be argued that the altered dentate gate function is associated with, but not essential for, epileptogenesis. Nonetheless, our data suggest that repetitive physiological activity could change the dynamics of the dentate granule cell responses in minislices from the epileptic brain and trigger epileptiform bursts, at least in some of the cases.
Interestingly, an earlier study (Finnerty et al. 2001) showed that repetitive stimulation of the perforant pathway at a frequency of 10–15 Hz [i.e., higher than theta frequencies (4–8 Hz)] caused depression in control slices (i.e., the so called “low-pass filtering” function of the dentate gyrus); however, in hippocampal slices prepared from tetanus toxin-injected rats, the responses of dentate granule cells potentiated after an initial depression during 15-Hz stimulation. Their data suggest that the dentate gyrus filtering function is altered in the tetanus toxin-injected rats. Thus in both the kainate-induced and tetanus toxin-induced epilepsy models, the dentate gate function seems to be compromised such that rhythmic physiological stimuli may trigger epileptiform activity or seizures. More recently, Bower and Buckmaster (2008) have shown that the firing rate of dentate granule cells is increased minutes before the onset of electrographic seizures, suggesting possible activity-dependent induction of seizures in the epileptic brain. However, we were unable to induce full-length seizure events under the current experimental conditions. Possibly slice preparations (particularly minislices) are less likely to generate prolonged seizure-like bursts because each minislice only contains a small fraction of the neural circuitry; the same stimulation protocol may cause full seizures in vivo. More in vivo and in vitro experiments are needed to further explore this issue.
Possible mechanisms for the induction of epileptiform bursts by repetitive stimulation
The induction of epileptiform bursts by repetitive stimulation suggests that it requires activity-dependent mechanisms, such as accumulation of extracellular K+, facilitation of synaptic excitation, and/or depression of synaptic inhibition (see following text). Several possible activity-dependent mechanisms might be involved in the induction of epileptiform bursts by repetitive stimulation. Most notably, the appearance of the epileptiform bursts was closely associated with and potentially resulted from the development of a negative DC shift and membrane depolarization (Fig. 4). Repetitive electrical stimulation is known to cause an accumulation of extracellular K+ (Heinemann et al. 1977; Krnjevic et al. 1982; Ransom et al. 2000; Stringer and Lothman 1989; Stringer et al. 1989) and a reduction of extracellular Ca2+ (Heinemann et al. 1977; Krnjevic et al. 1982), both of the changes would excite neurons and cause membrane depolarization and a negative DC shift of the field potential. In addition, repetitive stimulation may cause activity-dependent depression of GABAergic inhibition (McCarren and Alger 1985; Mott et al. 1993; Thompson and Gahwiler 1989a–c), which may further enhance and/or accelerate membrane depolarization and negative DC shift of field potentials. Moreover, GABAergic inhibition may be more prone to collapse in epileptic than normal dentate gyrus (Cohen et al. 2003; but see Molnar and Nadler 2001). Interestingly, however, while all of these changes may occur during repetitive stimulations in normal and epileptic slices, the negative DC shift in the field potential and the epileptiform bursts seldom occurred in controls, but occurred frequently in epileptic rats (sometimes even at lower frequency, i.e., 1–3 Hz, n = 3 slices, data not shown). Therefore, the propensity for the occurrence of a negative DC shift in the field potential, the membrane depolarization, and the epileptiform bursts were clearly increased in rats with kainate-induced epilepsy, possibly due to the increased number of recurrent excitatory synapses (Tauck and Nadler 1985; Wuarin and Dudek 1996) and enhanced probability of transmitter release (Scimemi et al. 2006) in this region. Moreover, the newly formed recurrent excitatory circuits during epileptogenesis may well serve as positive-feedback loops, such that the output of granule cells may be reinforced by previous stimulations and thus facilitate the generation of epileptiform bursts. All of these mechanisms may contribute an increased propensity for activity-dependent epileptiform bursts in slices from rats with kainate-induced epilepsy.
In summary, data from the present study suggest that the gate function of the dentate gyrus is significantly compromised in rats with kainate-induced epilepsy. The basis for this interpretation is that epileptiform bursts can be induced in a fraction (∼50%) of the dentate gyrus minislices by repetitive stimulation at a relatively physiological frequency, which in turn appears to be associated with membrane depolarization and a negative DC shift in the field potential. Additional experiments are needed to further explore whether or not full-length seizure events can be induced by repetitive stimulation in vivo and in vitro.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-16683.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present addresses: F. Edward Dudek, Dept. of Physiology, University of Utah School of Medicine, Salt Lake City, UT 84108; Li-Rong Shao, Dept. of Pharmacology, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814.
REFERENCES
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 14: 375–403, 1985 [DOI] [PubMed] [Google Scholar]
- Bertram EH. Temporal lobe epilepsy: where do the seizures really begin? Epilepsy Behav Suppl 1: 32–37, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Bower MR. Changes in granule cell firing rates precede locally recorded spontaneous seizures by minutes in an animal model of temporal lobe epilepsy. J Neurophysiol 99: 2431–2442, 2008 [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol 385: 385–404, 1997 [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Quirk GL, Coulter DA. Dentate granule cell GABA(A) receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci 17: 1607–1616, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE, Hellier JL, Williams PA, Ferraro DJ, Staley KJ. The course of cellular alterations associated with the development of spontaneous seizures after status epilepticus. Prog Brain Res 135: 53–65, 2002 [DOI] [PubMed] [Google Scholar]
- Finnerty GT, Whittington MA, Jefferys JGR. Altered dentate filtering during the transition to seizure in the rat tetanus toxin model of epilepsy. J Neurophysiol 86: 2748–2753, 2001 [DOI] [PubMed] [Google Scholar]
- Hardison JL, Okazaki MM, Nadler JV. Modest increase in extracellular potassium unmasks effect of recurrent mossy fiber growth. J Neurophysiol 84: 2380–2389, 2000 [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res 31: 73–84, 1998 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci 23: 2440–2452, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K, Morris ME, Reiffenstein RJ. Stimulation-evoked changes in extracellular K+ and Ca2+ in pyramidal layers of the rat's hippocampus. Can J Physiol Pharmacol 60: 1643–1657, 1982 [DOI] [PubMed] [Google Scholar]
- McCarren M, Alger BE. Use-dependent depression of IPSPs in rat hippocampal pyramidal cells in vitro. J Neurophysiol 53: 557–571, 1985 [DOI] [PubMed] [Google Scholar]
- Molnar P, Nadler JV. Mossy fiber-granule cell synapses in the normal and epileptic rat dentate gyrus studied with minimal laser photostimulation. J Neurophysiol 82: 1883–1894, 1999 [DOI] [PubMed] [Google Scholar]
- Molnar P, Nadler JV. Lack of effect of mossy fiber-released zinc on granule cell GABA(A) receptors in the pilocarpine model of epilepsy. J Neurophysiol 85: 1932–1940, 2001 [DOI] [PubMed] [Google Scholar]
- Mott DD, Xie CW, Wilson WA, Swartzwelder HS, Lewis DV. GABAB autoreceptors mediate activity-dependent disinhibition and enhance signal transmission in the dentate gyrus. J Neurophysiol 69: 674–691, 1993 [DOI] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci 13: 4470–4485, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, Dudek FE. Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J Neurophysiol 79: 418–429, 1998 [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32: 281–294, 1972 [DOI] [PubMed] [Google Scholar]
- Ransom CB, Ransom BR, Sontheimer H. Activity-dependent extracellular K+ accumulation in rat optic nerve: the role of glial and axonal Na+ pumps. J Physiol 522: 427–442, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A, Schorge S, Kullmann DM, Walker MC. Epileptogenesis is associated with enhanced glutamatergic transmission in the perforant path. J Neurophysiol 95: 1213–1220, 2006 [DOI] [PubMed] [Google Scholar]
- Shao LR, Dudek FE. Increased excitatory synaptic activity and local connectivity of hippocampal CA1 pyramidal cells in rats with kainate-induced epilepsy. J Neurophysiol 92: 1366–1373, 2004 [DOI] [PubMed] [Google Scholar]
- Stringer JL, Lothman EW. Maximal dentate gyrus activation: characteristics and alterations after repeated seizures. J Neurophysiol 62: 136–143, 1989 [DOI] [PubMed] [Google Scholar]
- Stringer JL, Williamson JM, Lothman EW. Induction of paroxysmal discharges in the dentate gyrus: frequency dependence and relationship to afterdischarge production. J Neurophysiol 62: 126–135, 1989 [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci 5: 1016–1022, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Activity-dependent disinhibition. I. Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J Neurophysiol 61: 501–511, 1989a [DOI] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Activity-dependent disinhibition. II. Effects of extracellular potassium, furosemide, and membrane potential on ECl− in hippocampal CA3 neurons. J Neurophysiol 61: 512–523, 1989b [DOI] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Activity-dependent disinhibition. III. Desensitization and GABAB receptor-mediated presynaptic inhibition in the hippocampus in vitro. J Neurophysiol 61: 524–533, 1989c [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rats. J Neurosci 16: 4438–4448, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurophysiol 85: 1067–1077, 2001 [DOI] [PubMed] [Google Scholar]