Fig. 1.
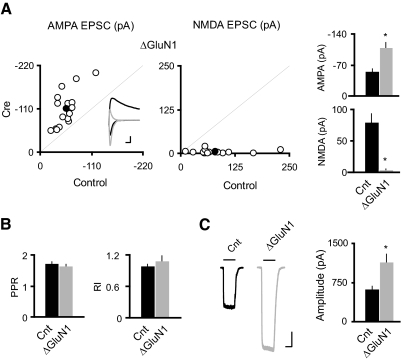
Single-cell genetic deletion of N-methyl-d-aspartate receptors (NMDARs) induced potentiation of both synaptic and extrasynaptic AMPA receptors (AMPARs) in CA1 pyramidal neurons. A: scatter plots and bar graphs at right show amplitudes of excitatory postsynaptic currents (EPSCs) for single pairs (open circles) and means ± SE (filled circles) from CA1 pyramidal neurons from acute slices (P17–P24) from GRIN1fl/fl mice injected with AAV-CRE/GFP at P0–P1. Inset: sample traces: black, control; gray, Cre. Scale bar, 40 pA, 0.02 s. EPSC amplitudes in the bar graphs show a significant enhancement in AMPAR EPSCs with the deletion of GluN1 (AMPA: Cnt, −55.75 ± 5.67 pA; ΔGluN1, −109.51 ± 11.49 pA; n = 16, *P < 0.0001; NMDA: Cnt, 80.15 ± 13.33 pA; ΔGluN1, 3.86 ± 1.24 pA, n = 16; *P < 0.0001). B: bar graphs show average paired-pulse ratio (PPR; Cnt: 1.73 ± 0.04, n = 84; ΔGluN1: 1.65 ± 0.05, n = 14; P = 0.39), and average rectification index (RI; Cnt: 0.99 ± 0.03, n = 30; ΔGluN1: 1.09 ± 0.10, n = 14, P = 0.21). C: sample traces of glutamate-evoked currents from outside-out patches in control (black) and Cre cells (gray). Bar graph shows that deletion of GluN1 led to ∼80% enhancement of the AMPAR-mediated outside-out patch currents (Cnt: −630.32 ± 44.68 pA; n = 24; ΔGluN1: −1146.10 ± 144.89 pA; n = 10; *P < 0.001). Scale bar, 200 pA, 1 s.