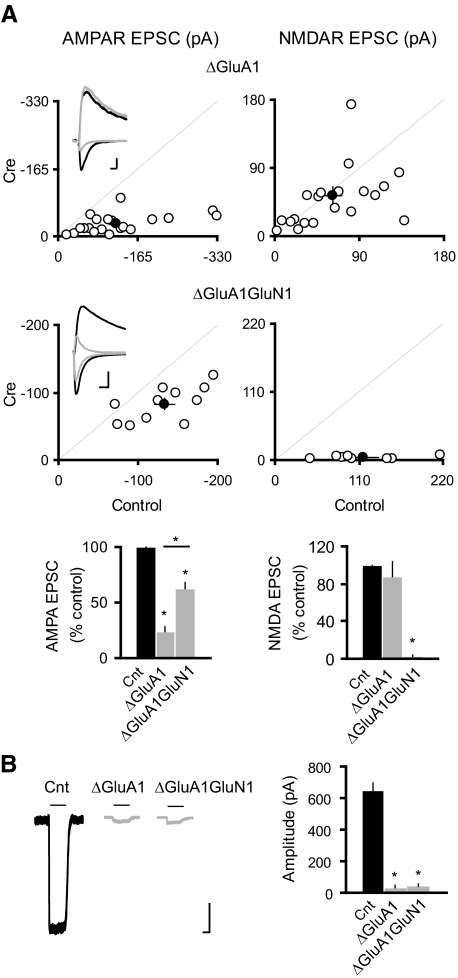
Fig. 2.
GluA1 subunit is not required for the NMDAR ablation-induced potentiation of synaptic AMPARs. A: scatter plots show amplitudes of EPSCs for single pairs (open circles) and means ± SE (filled circles) from CA1 pyramidal neurons from acute slices (P19–P25) from GRIA1fl/fl (top) and GRIA1fl/flGRIN1fl/fl (bottom) mice injected with AAV-CRE/GFP at P0–P1. Inset: sample traces: black, control; gray, Cre. Scale bar, 50 pA, 0.02 s. EPSC amplitudes in the bar graphs show a significant reduction in AMPAR EPSCs for the deletion of GluA1 or both GluA1 and GluN1 (ΔGluA1: −24.18 ± 4.30; n = 21, *P < 0.0001 as compared with control; ΔGluA1GluN1: −62.20 ± 5.90; n = 11; *P < 0.005 as compared with control). AMPAR EPSCs were significantly higher in ΔGluA1GluN1 than that in ΔGluA1 (*P < 0.0005). There was no change in the NMDAR EPSCs in ΔGluA1: 87.29 ± 16.90, n = 21; n = 21; P = 0.87; but NMDAR EPSCs were essentially eliminated in ΔGluA1GluN1: 3.10 ± 0.70, n = 8; *P < 0.0001). B: sample traces of glutamate-evoked currents from outside-out patches in control (black) and Cre cells (gray). Bar graph shows that deletion of GluA1 or both GluA1 and GluN1 eliminated the AMPAR-mediated outside-out patch currents (Cnt: −630.32 ± 44.68 pA; n = 24; ΔGluA1: −35.31 ± 13.05 pA, n = 16; ΔGluA1GluN1: −42.09 ± 16.66, n = 11; *P < 0.001 for both conditions). Scale bar, 100 pA, 1 s.