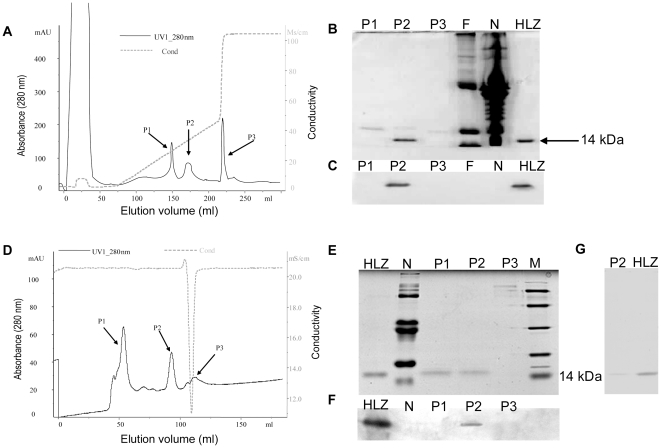
Figure 2. Purification of rHLZ by cation-exchange chromatography and gel filtration chromatography.
(A) Purification of rHLZ by cation-exchange chromatography. Transgenic milk whey (15 ml) was diluted in 20 mM sodium phosphate buffer, pH 8.5, and applied to the column. (B) The identification of rHLZ by 15% SDS-PAGE and Coomassie blue staining of the cation-exchange chromatography elution peaks. (C) Western blot identification of the elution peaks after cation-exchange chromatography. HLZ, commercial HLZ; N, milk from a non-transgenic cow; P1, P2 and P3 represent different elution peaks form cation-exchange chromatography; F represents the flow through. (D) Gel-filtration chromatography of the partly purified rHLZ from cation-exchange chromatography. The P2 fraction shown in A was loaded on the column after being concentrated with a centrifugal filter. (E) The identification of rHLZ by SDS-PAGE (15% gel) after Coomassie blue staining of elution peaks. HLZ, commercial HLZ; N, milk from a non-transgenic cow; M, protein marker; P1, P2 and P3 represent different elution peaks form Gel-filtration chromatography. (F) Western blot identification of SP Sepharose elution peaks. HLZ, commercial HLZ; N, milk from a non-transgenic cow; M, protein marker. P1, P2 and P3 represent different elution peaks form Gel-filtration chromatography. (G) The purified rHLZ protein after a two-step purification process was identified by SDS-PAGE (15% gel) and silver staining. HLZ, commercial HLZ. P2 represent elution peaks form Gel-filtration chromatography which is labeled in figure part D.