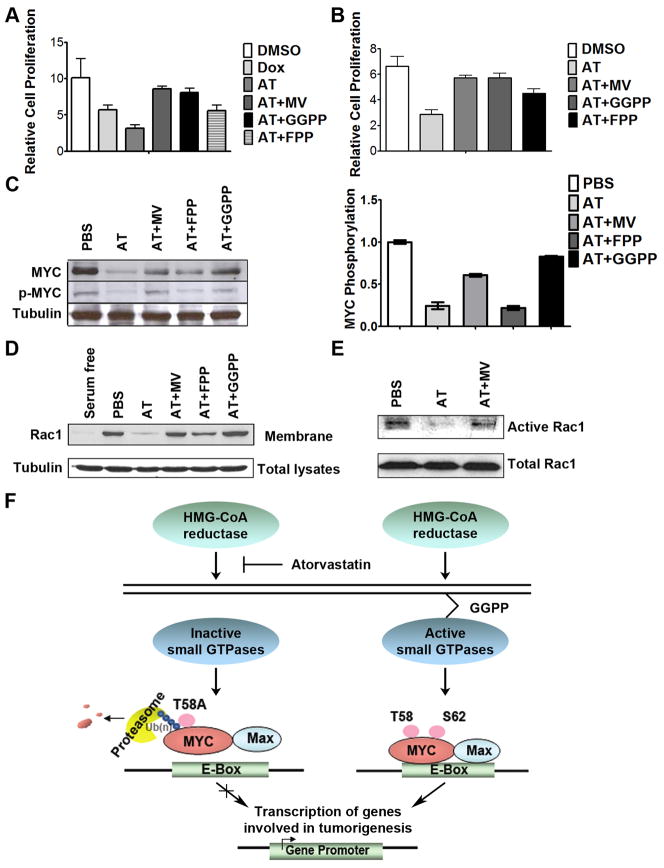
Figure 7. HMG-CoA reductase influences MYC phosphorylation through a Rac GTPase-dependent mechanism.
a) Suppression of murine HCC growth in vitro upon 10μM AT treatment for 96 hours is rescued by GGPP treatment, as assessed by MTT (DMSO versus AT+MV, p=0.07; DMSO versus GGPP, p=0.053, DMSO versus FPP, p=0.002). Error bars represent standard deviation. b) GGPP treatment rescues the AT- dependent suppression in human HCC cell growth upon 10μM AT treatment (DMSO versus AT+MV, p=0.01; DMSO versus GGPP, p=0.01, DMSO versus FPP, p<0.001). Error bars represent standard deviation. c) GGPP treatment rescues AT-dependent inhibition of MYC phosphorylation. Representative immunoblots are shown. Error bars represent standard deviation. d) GGPP treatment prevents the decrease in the membrane accumulation of Rac induced by 24 hours of 10μM AT. e) AT treatment inhibits Rac activity. Rac activity was reduced by 77% as measured by pull-down assay. f) Our results suggest a model in which inhibition of HMG-CoA reductase by AT blocks prenylation and activation of small GTPases, specifically including Rac. AT-mediated inhibition of Rac likely results in reduction of phospho-S62 MYC. Dephosphorylation at S62 in the context of phospho-T58 thereby results in the ubiquitin-mediated degradation of MYC. As such, AT treatment ultimately results in the inhibition of MYC oncogenic activity and suppressed hepatocellular carcinogenesis.