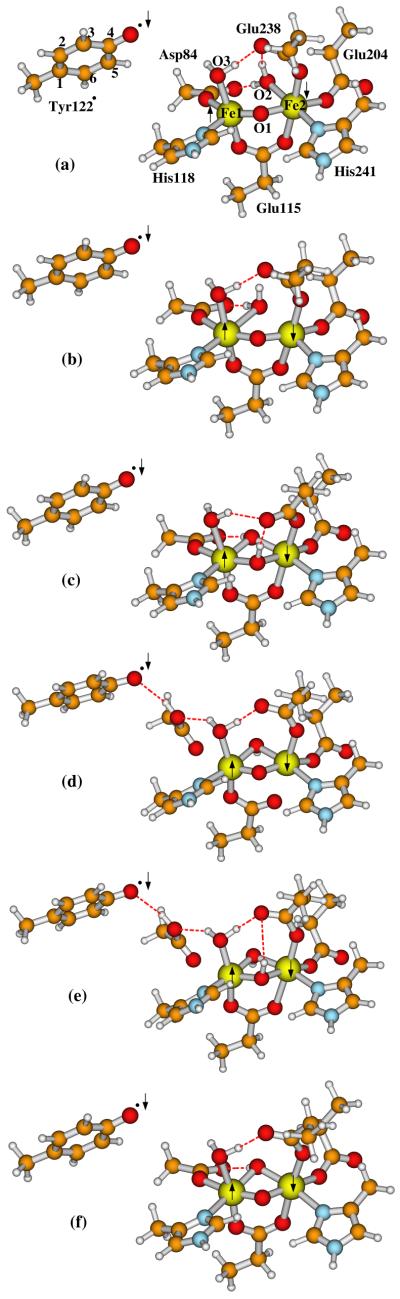
Figure 6.
Active site models for the active (A) state Fe1(III)Fe2(III)-Tyr122· of E. coli R2 studied in the current paper. The following names are given for these models and discussed in the text: (a) A(Obr,H2Ot2; (b) A(Obr,H2Ot1); (c) A(OH−br,OH−br); (d) A(Obr,OH−br)-Asp84H; (e) A(OH−br,OH−br)-Asp84H; and (f) A(Obr,OH−br), where “br” means the oxygen species is in the “bridging” position between Fe1 and Fe2, “t2” and “t1” in models (a) and (b) represent the ligand water molecule at site O2 “terminally” binding to Fe2 and Fe1, respectively, and Asp84H means the sidechain of Asp84 is protonated in that model. The arrows indicate the directions of the net spins on Fe1, Fe2, and Tyr122·. The outer-shell residue sidechains shown in Figure 2, including Gln43, Trp48, Trp111, Asp237, and water-627, are also included in calculations but not shown here.