Figure 6.
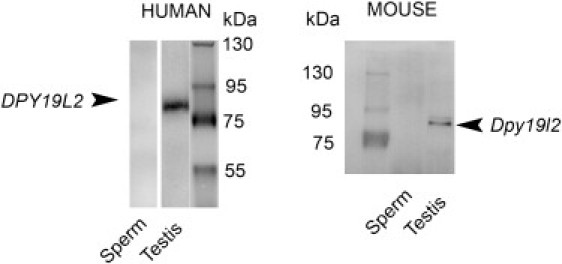
Immunoblots Showing the Presence of DPY19L2 in Testis and Its Absence in Sperm
Left; a unique band in the expected area of the gel is marked with human anti-DPY19L2 antibody with human testis extract but not with ejaculated sperm extract. A similar result is obtained with mouse anti-Dpy19l2 antibody, in which a band is marked with mouse testis extract but not with epididymal sperm extract . Protein loadings in each lane were similar and were checked by the Bradford protein assay.
Proteins were separated on 8% polyacrylamide denaturing gels and electrotransferred for 120 min at 350 mA to Immobilon P transfer membrane (Millipore). The membranes were then blocked for 60 min with 5% nonfat dry milk (Biorad) in PBS Tween 0.1%. The primary antibody was added and incubated overnight at 4°C. After washing in PBS Tween 0.1%, the secondary antibody (anti-rabbit, Jackson Laboratory) was added at a dilution of 1:10,000 for 1 hr at room temperature. The membrane was washed and incubated for 1 min in HRP substrate (Western Lightning, Perkin Elmer Life Science). The reactive proteins were detected with G-Box chemi XL (Syngene, England). Polyclonal antibodies against peptides from the N terminus of DPY19L2 (RSKLREGSSDRPQSSC for mouse Dpy19l2 and RSQSKGRRGASLAREPEC for human DPY19L2) were raised in rabbit. Antibodies were not purified, and serums were used at a dilution of 1:1000. All animal procedures were run according to the French guidelines on the use of living animals in scientific investigations with the approval of the local ethical review committee of Grenoble Neurosciences Institute. Sperm were obtained from 16-week-old Oncins France 1 strain (OF1) mice (obtained from Charles River, Macon, France) by manual trituration of caudae epididymis. Human testis tissue (from an 80-year-old donor) was obtained by surgery. Sperm were obtained by ejaculation (from a 30-year-old donor). Human tissues were obtained after approval by the local ethical committee and informed consent from the patients.
