Abstract
The antidiabetic activity of Pongamia pinnata ( Family: Leguminosae) leaf extracts was investigated in alloxan-induced diabetic albino rats. A comparison was made between the action of different extracts of P. pinnata and a known antidiabetic drug glibenclamide (600 μg/kg b. wt.). An oral glucose tolerance test (OGTT) was also performed in experimental diabetic rats. The petroleum ether, chloroform, alcohol and aqueous extracts of P. pinnata were obtained by simple maceration method and were subjected to standardization using pharmacognostical and phytochemical screening methods. Dose selection was made on the basis of acute oral toxicity study (50-5000 mg/kg b. w.) as per OECD guidelines. P. pinnata ethanolic extract (PPEE) and aqueous extract (PPAE) showed significant (P < 0.001) antidiabetic activity. In alloxan-induced model, blood glucose levels of these extracts on 7th day of the study were 155.83 ± 11.211mg/dl (PPEE) and 132.00 ± 4.955mg/dl (PPAE) in comparison of diabetic control (413.50 ± 4.752mg/dl) and chloroform extract (210.83 ± 14.912mg/dl). In glucose loaded rats, PPEE exhibited glucose level of 164.50 ± 6.350mg/dl after 30 min and 156.50 ± 4.089mg/dl after 90 min, whereas the levels in PPAE treated animals were 176 ± 3.724mg/dl after 30 min and 110.33 ± 6.687mg/dl after 90 min. These extracts also prevented body weight loss in diabetic rats. The drug has the potential to act as an antidiabetic drug.
Keywords: Acute toxicity, alloxan, antidiabetic activity, Pongamia pinnata
INTRODUCTION
Diabetes mellitus is a serious complex chronic condition that is a major source of ill health worldwide. This metabolic disorder is characterized by hyperglycemia and disturbances in carbohydrate, protein and fat metabolisms, secondary to an absolute or relative lack of the hormone insulin. Besides hyperglycemia, several other factors including dyslipidemia or hyperlipidemia are involved in the development of micro and macrovascular complications of diabetes, which are the major causes of morbidity and death.[1] According to World Health Organization (WHO) projections, the prevalence of diabetes is likely to increase 35% by 2020. Currently, there are over 150 million diabetics worldwide and this is likely to increase to 300 million or more by the year 2025. Statistical projection about India suggests that the number of diabetics will rise from 15 million in 1995 to 57 million in the year 2025, the highest number of diabetics in the world.[2] Reasons for this rise include increase in sedentary lifestyle, consumption of energy rich diet, obesity, higher life span, etc. Other regions with greatest number of diabetics are Asia and Africa, where diabetes mellitus rates could rise to 2–3 folds than the present rates.[3] Evaluation of plant products to treat diabetes mellitus is of growing interest as they contain many bioactive substances with therapeutic potential. In recent years, several authors have reported the antidiabetic potential of traditionally used Indian medicinal plants using experimental animals.[4–9] Although a large number of medicinal plants have been already tested for their antidiabetic effects, several other Indian medicinal plants remain to be investigated.[10]
Pongamia pinnata (Family: Leguminosae) is a medium-sized, glabrous, semi-evergreen tree, growing up to 18 m or higher, with a short bole, spreading crown with grayish green or brown bark. Leaves are imparipinnate, alternate, and leaflets are 5–7 in number, ovate in shape and opposite in arrangement. This tree is popularly known as Karanja in Hindi, Indian Beech or Derris indica in English, and Hongae in Kannada. P. pinnata occurs all over India in the bank of rivers streams and planted as an avenue tree in gardens. The leaves of P. pinnata have been used in Ayurvedic medicine as digestive, laxative, anthelmintic, to cure piles, wound healing, relieving rheumatic pains, for cleaning ulcers in gonorrhea and scrofulous enlargement. Previous studies have demonstrated that P. pinnata is rich in flavonoids and related compounds. Seeds and seed oil, flowers and stem bark yield karanjin, pongapin, pongaglabrone, kanugin, desmethoxykanugin and pinnatin.[11] Furanoflavonoid glucosides (pongamosides A–C) and flavonol glucoside (pongamoside D) have also been reported.[12]
The rationale behind using Karanja (P. pinnata) in prameha is due to its katu rasa, katu vipaka, tikshna, ushna and deepana and pachana properties. According to Bhavaprakasha Nighantu by Sri Bhava Misra in Guduchyadivarga, the leaves of karanja are said to be kapha-vata nashak, krimighna and shothaghna and fruits are considered as prameghna.[13] Since the fruit of this plant is reported to be prameghna (antidiabetic) and Ayurvedic properties of fruits and leaves are similar, its leaves can possibly have antidiabetic potential.
To the best of our knowledge, no scientific data regarding the antidiabetic effect of P. pinnata leaves (although fruits and flowers have been investigated)[14–16] are available except in the treatise of Ayurvedic medicine. Thus, the present study was undertaken to evaluate the antidiabetic effect of P. pinnata leaves in alloxan-induced diabetic rats.
MATERIALS AND METHODS
Animals
Adult albino rats of Wistar strain (150–200 g) of either sex were procured from Government Veterinary College, Bangalore, and were housed in the animal house of K. L. E. S College of Pharmacy, Ankola, with 12 hr light and 12 hr dark cycles. Standard pellets obtained from Goldmohar rat feed, Mumbai, India, were used as a basal diet during the experimental period. The control and experimental animals were provided food and drinking water ad libitum. All the animal experiments were conducted according to the ethical norms approved by CPCSEA, Ministry of Social Justice and Empowerment, Government of India, and ethical clearance was granted by Institutional Ethical Committee in resolution no. 1/18/2007 held on 23 November 2007 at JN Medical college, Belgaum (ethical committee IAEC reg. no.: 627/02/a/CPCSEA).
Chemicals
Following is the list of chemicals used.
Alloxan monohydrate (Spectrochem Pvt. Ltd., Mumbai), Glibenclamide (Aventis Pharma Ltd., Verna, Goa), Dextrose (Emkay Labs, India), Tween 80 (S. D. Fine-chem limited, Mumbai), Anesthetic Ether (Ozone International, Mumbai).
Accu-chek® Active Glucometer, Roche Diagnostic Corporation, Germany. Blood gluco-strips (Roche Diagnostic Pvt. Ltd., Mumbai, India) . All other chemicals and reagents used were of analytical grade.
Plant material
Leaves of P. pinnata were collected in and around the local forest area of Sirsi in Western Ghats, Karnataka, and authenticated by the Botanist Prof. G. S. Naik, Department of Botany, G. C. Science and Art College, Ankola. A voucher herbarium specimen number GCSAC/PP/01 was also preserved in the same college. The collected leaves were dried under shade at room temperature (25°C) for 10 days and powdered to a coarse consistency in a grinder mill. The powder was passed through 40 # mesh particle size and stored in an airtight container at room temperature.
Preparation of plant extract
2.5 kg of the fresh, air-dried, powered crude drug of P. pinnata was extracted with petroleum ether (60-80°C), chloroform, 95% ethanol and chloroform water (i.p.) by adopting simple maceration procedure at room temperature for 7 days in a conical flask with occasional shaking and stirring. The extract was filtered and concentrated to dryness at room temperature to avoid the decomposition of the natural metabolites.[17] The yield of the extracts was 1.79%, 4.63%, 20.86% and 18.54% w/w for petroleum ether, chloroform, ethanol and water, respectively. All the extracts were preserved in a refrigerator till further use. Preliminary phytochemical analysis was carried out in all four extracts by different methods of phytochemical analysis.[18] A known volume of extract was suspended in distilled water and was orally administered to the animals by gastric intubation using a gavage during the experimental period.
Acute oral toxicity studies
The acute oral toxicity studies[19] of extracts were carried out as per the OECD guidelines, draft guidelines 423 adopted on 17 December 2001 received from CPCSEA, Ministry of Social Justice and Empowerment, Government of India. Administration of the stepwise doses of all four extracts of P. pinnata from 50 mg/kg b. wt. up to a dose of 5000 mg/kg b. wt. caused no considerable signs of toxicity in the tested animals. One-tenth of the upper limit dose was selected as the level for examination of antidiabetic activity.
Experimental models
Oral glucose tolerance test[20]
Fasted rats were divided into six groups of six rats in each. Group I served as normal control and received distilled water with Tween 80. Group II received standard drug Glibenclamide as an aqueous suspension at a dose of 600 μg/kg b. wt. Groups III-VI received the different extracts at a dose of 500 mg/kg b. wt. as a fine Tween 80 suspension. After 30 min of extract administration, the rats of all groups were orally treated with 2 g/kg of glucose. Blood samples were collected from the rat tail vein just prior to glucose administration and at 30, 60 and 90 min after glucose loading. Blood glucose levels were measured immediately by using Glucometer.
Alloxan-induced diabetic model[21]
Alloxan monohydrate was first weighed individually for each animal according to its weight and then solubilized with 0.2 ml saline just prior to injection. Diabetes was induced by injecting it at a dose of 150 mg/kg b. wt. intraperitonially.[11] After 1 hr of alloxan administration, the animals were given feed ad libitum and 5% dextrose solution was also given in feeding bottle for a day to overcome the early hypoglycemic phase. The animals were kept under observation, and after 48 hr, blood glucose was measured by glucometer. One group served as a control which received vehicle alone. The diabetic rats (glucose level > 300 mg/dl) were separated and divided into six different groups for experimental study, with each group containing six animals. Group II were left untreated and served as diabetic controls. Group III received Glibenclamide 600 μg/kg, Group IV rats were treated with aqueous extract of P. pinnata (PPAE) at a dose of 500 mg/kg b. wt., Group V received ethanolic extract (PPEE) of P. pinnata at a dose of 500 mg/kg b. wt., Group VI rats were treated with chloroform extract of P. pinnata (PPCE) 500 mg/kg b. wt. and Group VII diabetic rats were treated with petroleum ether extract of P. pinnata (PPPEE) 500 mg/kg b. wt. for 7 days.
Body weight, urine sugar and lipid profile measurement
Body weight, urine sugar and lipid profile[22] of diabetic rats were measured during the course of study period [i.e., before alloxan induction (initial values), on the 1st and 7th days of the treatment period]. After the 7th day of treatment, blood was collected retro-orbitally (under light ether anesthesia) using capillary tubes in fresh vials containing sodium fluoride and sodium oxalate as anticoagulant agents. The serum was separated by using centrifuge at 2000 rpm for 2 min. Total cholesterol (TC), triglyceride (TG) and high density lipoprotein (HDL) were analyzed using diagnostic kits (Span diagnostic Ltd., Surat, Gujarat, India) using colorimeter. Low density lipoprotein (LDL) and very low density lipoprotein (VLDL) levels were calculated using the formula of Friedewald et al.[23]Urine sugar was detected with uristix.
Statistical analysis
The results of the study were subjected to one-way analysis of variance (ANOVA) followed by Dunnett's t-test for multiple comparisons. Values with P <0.05 were considered significant.
RESULTS
Standardization and phytochemical screening
Standardization parameters for P. pinnata leaves were determined and all the parameters were found to be within pharmacopoeial standards limit. Crude powder taken for extraction was of green color with slight bitter taste. Losses on drying, total ash, acid insoluble ash and water soluble ash were 3.67%, 6.35%, 3.54% and 1.05% w/w, respectively.
Thin layer chromatography of P. pinnata leaves revealed yellow/orange spots/florescence with Rf values 0.56, 0.72, 0.43, 0.25, and 0.86. Phytochemical screening of all the extracts of P. pinnata showed the presence of various phytochemical constituents like flavonoids, triterpenoids, carbohydrates, tannins, phytosterols and traces of alkaloids.
Toxicity study
In acute toxicity study, none of the studied extracts of P. pinnata leaves showed any significant toxicity sign when observed for the parameters during the first 4 hrs and followed by daily observations for 14 days and mortality was also not observed; the drug was found to be safe at the tested dose level of 5000 mg/kg b. w. One-tenth of this dose level was taken as effective dose. All the extracts were experimented at the same dose of 500 mg/kg b. w. Since the yield was less with petroleum ether and chloroform extract and possibility of active compounds was also lesser in such yield . Reason for this may be simple maceration procedure used for extraction in which the solvent does not penetrate into the plant cells too well and adequate amount of active compound does not come out in extract; if yield is too less, selecting lesser doses may be ineffective and higher doses may be toxic and noncompliant.
In order to ascertain a scientific base for the usefulness of this plant in the treatment of diabetes, it was decided to evaluate experimental design of antidiabetic activity by following glucose tolerance test and alloxan-induced model.
Alloxan-induced diabetic model
As expected in the diabetic control, there was severe hyperglycemia as compared to the normal animals. Compared to the diabetic control, all the four extracts (PPAE, PPEE, PPCE and PPPEE) lowered the elevated blood glucose levels only in subacute treatment [Table 1]. It was observed that the standard drug glibenclamide lowered the blood glucose level significantly, bringing it nearly back to normal, whereas PPEE and PPAE significantly (P < 0.01) decreased fasting blood serum glucose in the diabetic rats on 3rd, 5th and 7th days as compared to initial (0 hr) blood serum glucose levels [Figure 1]. When PPEE and PPAE extracts of P. pinnata were compared for their antidiabetic activity in comparison to active control, particularly Glibenclamide, the results showed that their potential was lesser but significant (**P < 0.01) than the standard drug at subacute level.
Table 1.
Effect of Pongamia pinnata extracts on blood glucose level of alloxan-induced diabetic albino rats after subacute treatment
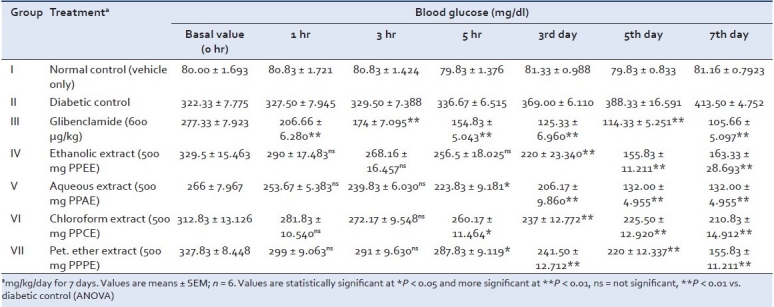
Figure 1.
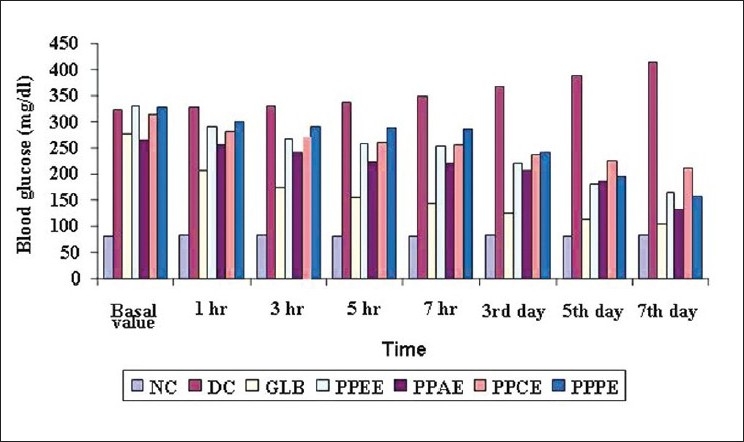
Blood glucose level of alloxan-induced diabetic albino rats after treatment
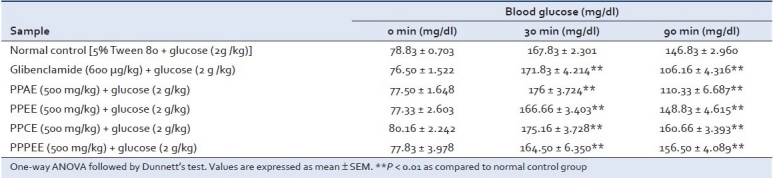
Oral glucose tolerance test model
The effect of different extracts on glucose tolerance test in normal rats is shown in Table 2. At 30 min after glucose administration, the peak of blood glucose level increased rapidly from the fasting value and then subsequently decreased. The aqueous (PPAE) and ethanolic (PPEE) extracts of the leaves of P. pinnata exhibited remarkable blood glucose lowering effect at 90 min.
Table 2.
Effect of Pongamia pinnata extracts on blood glucose level in oral glucose tolerance test in normal rats
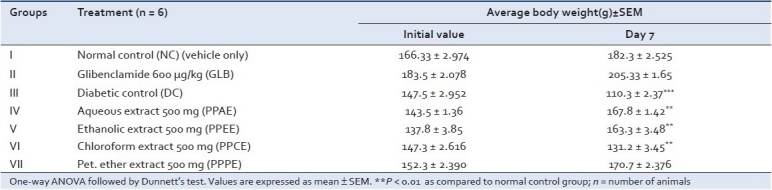
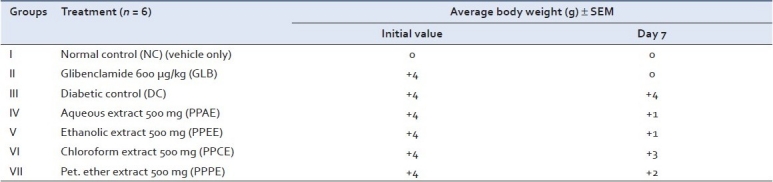
Body weight
In the present study, diabetic rats had lower body weights and high blood glucose level as compared to normal rats. In spite of increased food consumption, loss of body weight due to defect in glucose metabolism and excessive breakdown of tissue protein is a characteristic condition in diabetics. As shown in Table 3, treatment with PPAE and PPEE improved the average body weights of rats, which indicates that control over polyphagia and muscle wasting resulted due to hyperglycemic condition.
Table 3.
Effect of various extracts of Pongamia pinnata on body weight after treatment in diabetic rats
Urine sugar and lipid profile
It was intended to assess the effect of long-term treatment on blood glucose level, urine sugar and associated abnormal lipid profile in alloxan-induced severely diabetic rats. The various parameters of blood lipid profile of severely diabetic rats were estimated before and after 7 days of treatment [Table 4]. The enhanced levels of TC, LDL cholesterol and TG were brought down significantly (P < 0.001) after 14 days of treatment. A fall of 70% urine sugar was observed after 14 days of treatment [Table 5]. This may be due to improvement in the glycemic control mechanisms and insulin secretion from remnant pancreatic β-cells in diabetic rats.
Table 4.
Effect of oral administration of the various extracts of Pongamia pinnata on serum lipid profile in severe diabetic rats (mean ± SD)
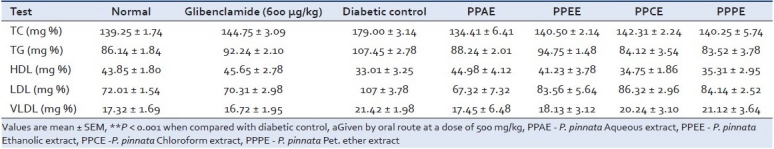
Table 5.
Effect of various extracts of Pongamia pinnata on urine sugar before and after treatment in diabetic rats
DISCUSSION
Diabetes mellitus, a common heterogeneous metabolic syndrome, is prevalent throughout the world and has been projected to become one of the world's main disablers and killers within the next 25 years. Blood glucose level, urine sugar and body weight have been commonly measured to monitor the glycemic control mechanism. In the present study, diabetic rats had lower body weight, high blood and urine sugar levels as compared to normal rats. However, orally administered PPAE and PPEE significantly increased the body weight and decreased the blood glucose level. This could be due to potentiation of the insulin effect of plasma by increasing the pancreatic secretion of insulin from existing β-cells of islets of Langerhans or its release from bound insulin. The significant and consistent antidiabetic effect of PPAE and PPEE in alloxan diabetic rats may also be due to enhanced glucose utilization by peripheral tissues.
Several authors reported flavonoids, sterols, alkaloids and polyphenols as bioactive antidiabetic principles. The phytochemical screening of P. pinnata revealed the presence of various flavonoids, furoflavones, triterpenoids, carbohydrates, tannins, phytosterols and other polyphenolic compounds. Hence, the antidiabetic activity of the above-mentioned PPAE and PPEE is probably due to the presence of several bioactive antidiabetic principles and their synergistic properties.
The levels of serum lipids are usually elevated in diabetes mellitus and such an elevation represents a risk factor for coronary heart diseases. The marked hyperlipidemia that characterizes the diabetic states may be regarded as a consequence of the uninhibited actions of lipolytic hormones on the fat depots. Lowering of serum lipid concentration through dietary or drug therapy seems to be associated with a decrease in the risk of vascular diseases. The result of this study reveals that the dose of 500 mg/kg not only lowered TC, TG and LDL, but also enhanced the cardioprotective lipid HDL. The fall of 50% and 75% urine sugar of severely diabetic group after 7 days of treatment with the most effective dose further confirms our findings.
CONCLUSION
Alloxan, a β-cytotoxin, causes a massive destruction of β-cells of the islets of Langherhans, resulting in reduced synthesis and release of insulin. The function of the insulin system is suppressed, which leads to high level of hyperglycemia and eventually to death, but PPAE and PPEE showed potent antidiabetic effect in alloxan-induced diabetic rats and reduced the mortality rate significantly. The present investigation has also opened avenues for further research, especially with reference to the different dose studies and development of potent formulation for diabetes mellitus from P. pinnata leaves. Activity guided fractionation, formulation and its evaluation is in progress and will be available in a short period of time.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Apparao C, Kameswararao B, Kesavulu MM. Evaluation of antidiabetic effect of Momordica cymbalaria fruit in alloxan-diabetic rats. Fitoterapia. 2003;74:7–13. doi: 10.1016/s0367-326x(02)00297-6. [DOI] [PubMed] [Google Scholar]
- 2.Satyanarayana T, Katyayani BM, Hemalatha E, Anjana AM, Chinna EM. Hypoglycemic and antihyperglycemic effect of alcoholic extract of Euphorbia leucophylla and its fractions in normal and in alloxan induced diabetic rats. Pharmacogn Mag. 2006;2:244–53. [Google Scholar]
- 3.Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13:624–9. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Shirwaikar A, Rajendran K, Punitha IS. Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in streptozotocin-nicotinamide induced type 2 diabetic rats. J Ethnopharmacol. 2005;97:369–74. doi: 10.1016/j.jep.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Maroo J, Vasu VT, Gupta S. Dose dependent hypoglycemic effect of aqueous extract of Enicostemma littorale Blume in alloxan induced diabetic rats. Phytomedicine. 2003;10:196–9. doi: 10.1078/094471103321659933. [DOI] [PubMed] [Google Scholar]
- 6.Chhetri DR, Parajuli P, Subba GC. Antidiabetic plants used by Sikkim and Darjeeling Himalayan tribes, India. J Ethnopharmacol. 2005;99:199–202. doi: 10.1016/j.jep.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 7.Shankar PK, Kumar Vasanth, Rao Namita. Evaluation of Antidiabetic Activity of Ginkgo Biloba in Streptozotocin Induced Diabetic Rats. Iranian Journal of Pharmacology and Therapeutics. 2005;4:16–9. [Google Scholar]
- 8.Grover JK, Yadav SP. Pharmacological actions and potential uses of Momordica charantia: A review. J Ethanopharmacol. 2004;93:123–32. doi: 10.1016/j.jep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Shirwaikar A, Rajendran K, Barik R. Effect of aqueous bark extract of Garuga pinnata Roxb. J Ethnopharmacol. 2006;107:285–90. doi: 10.1016/j.jep.2006.03.012. In streptozotocin-nicotinamide induced type-II diabetes mellitus. [DOI] [PubMed] [Google Scholar]
- 10.Manoharan S, Punitha R. Antihyperglycemic and antilipidperoxidative effects of Pongamia pinnata (Linn.) Pierre flowers in alloxan induced diabetic rats. J Ethnopharmacol. 2006;105:39–46. doi: 10.1016/j.jep.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 11.Joy PP, Thomas J, Mathew S, Skaria BP. Medicinal Plants.Kerala India: Agricultural university research station publishers. 1998:73. [Google Scholar]
- 12.Maurya R, Ahmad G, Yadav PP. Furanoflavonoid glycosides from Pongamia pinnata fruits. Phytochemistry. 2004;65:921–4. doi: 10.1016/j.phytochem.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Bhavamisra . Bhavaprakash Nighantu commentary by Chunekar KC. In: Pandey GS, editor. Guduchyadi varga sloka 121,122. 7th ed. Varanasi (India): Chaukhamba Bharati Academy; 2006. p. 350. [Google Scholar]
- 14.Punitha R, Manoharan S. Antihyperglycemic and antilipidperoxidative effects of Pongamia pinnata (Linn.) Pierre flowers in alloxan induced diabetic rats. J Ethnopharmacol. 2006;105:39–46. doi: 10.1016/j.jep.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 15.Punitha R, Vasudevan K, Manoharan S. Effect of Pongamia pinnata flowers on blood glucose and oxidative stress in alloxan induced diabetic rats. Indian J Pharmacol. 2006;38:62–3. [Google Scholar]
- 16.Tamrakar AK, Yadav PP, Tiwari P, Maurya R, Srivastav AK. Identification of pongamol and karanjin as lead compounds with antihyperglycemic activity from Pongamia pinnata fruits. J Ethnopharmacol. 2008;118:435–9. doi: 10.1016/j.jep.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Pharmacopoeia of India. Ministry of Health New Delhi: Government of India. 1982:948–650. [Google Scholar]
- 18.Khandewal KR. 14th ed. Pune: Nirali prakashan; 2005. Practical Pharmacognosy; pp. 146–57. [Google Scholar]
- 19.OECD/OCDE, OECD Guidelines for the testing of chemicals, revised draft guidelines 423: Acute Oral toxicity- Acute toxic class method, revised document, CPCSEA, Ministry of Social Justice and Empowerment. 2000 New Delhi: Government of India.
- 20.Sellamuthu PS, Muniappan BP, Perumal SM, Kandasamy M. Antihyperglycemic effect of mangiferin in streptozotocin induced diabetic rats.J. Health Sci. 2009;55:206–14. [Google Scholar]
- 21.Kannur DM, Hukkeri VI, Akki KS. Antidiabetic activity of Caesalpinia bonducella seeds extracts in rats. Fitoterapia. 2006;77:546–9. doi: 10.1016/j.fitote.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Nagappa AN, Thakurdesai PA, Rao VN, Jiwan Singh. Antidiabetic activity of Terminalia catappa linn fruits. J Ethanopharmacol. 2003;88:45–50. doi: 10.1016/s0378-8741(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]


