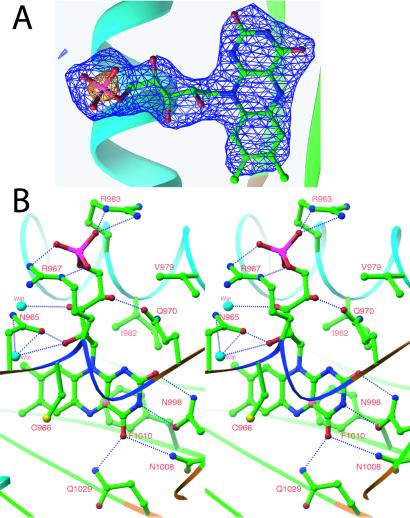
Figure 2.
Chromophore of phy3 LOV2. (A) Simulated-annealing omit map of FMN from one of the four monomers in the asymmetric unit. The map is contoured at ±3.5σ (blue) and ±10σ (yellow), in which σ is the rms-deviation value of the electron density. Electron density distinguishes the dimethylbenzene and pyrimidine moieties of the isoalloaxazine ring and shows a +10σ feature over the terminal phosphate. (B) Stereo diagram of FMN–protein interactions. All residues and waters that hydrogen bond to or form van der Waals contact with FMN are shown. Hydrogen bonds are indicated by the dotted blue lines using a 2.6- to 3.5-Å range for hydrogen bonding. Atoms are colored as in Fig. 1 with the addition of sulfur as yellow, water molecules as light blue, and C(4a) of the isoalloxazine ring as pink.