Abstract
Regular physical activity (endurance training, ET) has a strong positive link with cardiovascular health. The aim of this review is to draw together the current knowledge on gene expression in different cell types comprising the vessels of the circulatory system, with special emphasis on the endothelium, and how these gene products interact to influence vascular health. The effect beneficial effects of ET on the endothelium are believed to result from increased vascular shear stress during ET bouts. A number of mechanosensory mechanisms have been elucidated that may contribute to the effects of ET on vascular function, but there are questions regarding interactions among molecular pathways. For instance, increases in flow brought on by ET can reduce circulating levels of viscosity and haemostatic and inflammatory variables that may interact with increased shear stress, releasing vasoactive substances such as nitric oxide and prostacyclin, decreasing permeability to plasma lipoproteins as well as the adhesion of leucocytes. At this time the optimal rate-of-flow and rate-of-change in flow for determining whether anti-atherogenic or pro-atherogenic processes proceed remain unknown. In addition, the impact of haemodynamic variables differs with vessel size and tissue type in which arteries are located. While the hurdles to understanding the mechanism responsible for ET-induced alterations in vascular cell gene expression are significant, they in no way undermine the established benefits of regular physical activity to the cardiovascular system and to general overall health. This review summarizes current understanding of control of vascular cell gene expression by exercise and how these processes lead to improved cardiovascular health.
Keywords: blood flow, capillarity, endothelium, gene expression, vascular smooth muscle
Physical inactivity is an independent risk factor for coronary artery disease, one of the world’s leading causes of death (Beaglehole et al. 2007). Protection from the complications of vascular disease can be gained from a lifelong pattern of physical activity, but beginning an exercise programme at any stage of life can yield significant cardiovascular health benefits (Grau et al. 2009, Walker et al. 2009). Even in individuals who have experienced a cardiac event, evidence confirms that exercise training rehabilitation reduces the extent of disability, enhances quality of life, and positively influences morbidity and mortality (Piotrowicz & Wolszakiewicz 2008). The direct relationship between exercise and vascular health is certain, but the complex set of metabolic pathways, haemodynamic effects of exercise on cardiovascular cells/tissues, and the regulation of genetic expression activated by exercise is still largely undefined (Laughlin et al. 2008). An accompanying chapter will discuss the effects of acute and chronic exercise on the heart. The aim of this review is to draw together the current knowledge on gene expression in different cell types comprising the vessels of the circulatory system, with special emphasis on the endothelium, and how these gene products interact to influence vascular health under conditions of rest, acute and chronic exercise. Excellent reviews on the genetics of cardiovascular health and the multiple signalling mechanisms in tissues (e.g. skeletal muscle) in response to exercise have been published (Govindaraju et al. 2008, Röckl et al. 2008). However, this field is in a state of constant flux due to the rapid rate at which new information is becoming available. The human genome project has provided an abundance of information, and emerging technologies such as massively parallel DNA sequencing now enable the complete human genome of unique individuals to be deciphered in 1–2 months, generating data at a rate of 4–8 Gb per week (Wang et al. 2008, Wheeler et al. 2008). Interpreting the regulation, expression and physiological effects of the products of these genes on health and function continues to be a rapidly evolving area of research. By focusing on the gene products specific to cells of the vasculature, our hope is that this document will provide a synthesis of our current understanding of how physical activity influences gene expression, both in healthy trained individuals and in those with various cardiovascular disease states. Because much of this work has been done examining the effects of chronic exposure to increased physical activity, more space is committed to the effects of long-term physical activity on gene expression. However, the effect of acute bouts of exercise on vascular cell gene expression is an area of increasing activity and interest in the field, so this will be discussed as well. At some level, chronic effects of physical activity on gene expression reflect sustained effects of changes in gene expression during/following single bouts of exercise. We will focus not only on endogenous vascular gene expression but also on new information gained from areas such as transgenics, gene therapy and pluripotent progenitor cells.
The vascular response during acute bouts of exercise
Under resting conditions, the heart provides sufficient cardiac output to sustain basal metabolic needs and the central cardiovascular reflexes work to maintain blood pressure in the normal range. Within each tissue, the resistance arteries determine regional peripheral resistance in order to provide adequate blood supply to meet the metabolic demands of the body. Vascular resistance is determined by the calibre of the resistance arteries which is controlled by the level of contraction of the vascular smooth muscle (VSM) surrounding the arteries. These arteries have a level of basal tone (basal level of contraction of the VSM) and are also influenced by central control signals (sympathetic constriction) and local chemical and mechanical factors. Thus, VSM in these resistance arteries serves as the integrator of these many inputs that establish vascular resistance in the tissue in which the resistance arteries reside.
Acute initiation of muscle contraction driving physical activity (i.e. aerobic or dynamic exercise) raises the muscular requirement for nutrients and oxygen. Within the skeletal muscle vascular control mechanisms provide a nearly linear increase in blood flow, which together with alterations in oxygen extraction matches the increase in oxygen consumption of the muscle. Central control processes enable a near-linear increase in heart rate and cardiac output that are also matched to oxygen consumption of the muscle tissue (reviewed by Laughlin 1999). Increased cardiac output is a direct response to the volume increase in resistance vessels (vasodilation) to meet the oxygen demand of the exercising skeletal and cardiac muscles (Duncker & Bache 2008). The increase in cardiac output is supplied by enhanced venous return, the result of a decrease in the splanchnic or visceral blood flow and to the compressive effects of muscle contraction on muscle veins (the muscle pump; Osada et al. 1999). Vascular resistance is the main control mechanism for blood flow during exercise, and this resistance is controlled at the local vascular level of the muscle tissue by altering VSM contraction in the resistance arteries (Delp & Laughlin 1998, Thomas & Segal 2004). During exercise there continues to be an effect of the central cardiovascular reflexes maintaining systemic blood pressures. There are complex interactions of vasodilating signals and vasoconstrictor signals in the VSM of the resistance arteries in active skeletal muscle. Under most circumstances the net effect is that central sympathetic stimulation is counterbalanced by reduction in total peripheral resistance of the skeletal muscle vasculature. Even a fivefold increase in cardiac output among normal subjects during exercise only moderately increases mean arterial pressure (Engel & Froelicher 2008).
Local control of blood flow
The local control of muscle blood flow during exercise is generally considered to be mediated by the release of chemicals (metabolites and other local vasoactive compounds) from the active muscle in proportion to metabolic rate, to mechanical stimulation of arteries supplying the muscles (Clifford 2007, Laughlin & Roseguini 2008), vasodilation activated in the smallest arterioles that is conducted upstream of larger arterioles and resistance arteries, and paracrine signalling from endothelial cells (ECs) and red blood cells (Clifford & Hellsten 2004, Fig. 1). Changes in intravascular pressure during exercise result in a triphasic change in intraluminal pressure referred to as the myogenic response (Fig. 1A). Initially (1) arterioles dilate passively with increasing pressure until (2) a range from 20 to 120 mmHg elicits myogenic constriction. Beyond these pressures (3) (>140 mmHg), any additional rise in pressure promotes increased dilation (recently reviewed by Carlson et al. 2008). Relaxation of VSM can also be induced by the conducted vasodilation response (Fig. 1B), where capillaries exhibit the capacity to respond directly to metabolic inputs with a signal that communicates with the upstream arterioles, enabling capillaries to control their own perfusion (Murrant & Sarelius 2000). This response, perhaps stimulated by acetylcholine (ACh), is characterized by the initiation and spread of hyperpolarizing current along the endothelium and into surrounding smooth muscle cells through gap junctions (Yashiro & Duling 2003, Domeier & Segal 2007). This hyperpolarization closes voltage-gated calcium (Ca2+) channels, producing a nearly instantaneous relaxation of smooth muscle along the vessel due to the drop in intracellular Ca2+ (Ledoux et al. 2006). Although this response can be reproduced in isolated vessels, the relative importance of endogenous ACh released from neuromuscular junctions in exercise-induced hyperaemia (e.g. human leg) may not be as great as other mechanisms of vasomotor control (Hellsten et al. 2009).
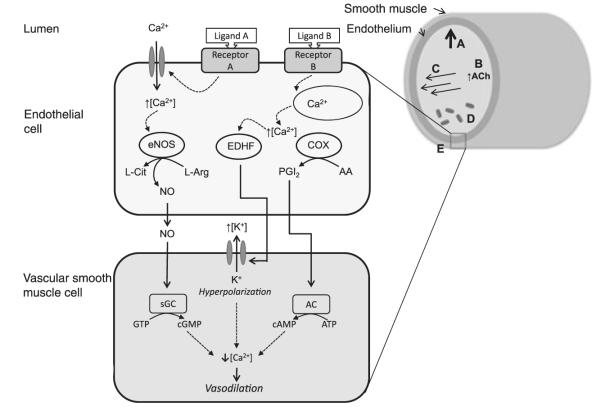
Figure 1.
Local control of arterial blood flow in skeletal muscle during exercise (see text for details). (A) Myogenic response: blood pressure results in stretching of the vessel wall circumference. (B) Conducted response: vascular signalling by release of acetylcholine, for example. Other putative signalling involves KATP channels, although their location (on endothelial, vascular smooth muscle or skeletal muscle cells) is not yet defined (Murrant & Sarelius 2000). (C) Flow-mediated response: fluid shear stress due to longitudinal blood flow. (D) Metabolic response: chemical release by red blood cells and skeletal muscle, generating an upstream vasomotor signal. (E) Endothelial-mediated responses at the cellular level (image not to scale). NO, nitric oxide; eNOS, endothelial nitric oxide synthase; PGI2, prostacyclin; ACh, acetylcholine; sGC, soluble guanylate cyclase; EDHF, endothelium-derived hyperpolarizing factor; COX, cyclooxygenase; AC, adenylate cyclase.
The luminal surface of all arteries and veins is lined with a single layer of ECs, the endothelium. ECs normally control the exchange of substances between the blood and tissues and serve as sensors of physical and chemical signals from the blood. ECs are far more important than just a lining to the blood vessels. The endothelium is known to regulate vascular permeability, participate in haemostasis, mediate immune and inflammatory responses, regulate leucocyte and platelet adhesion, modulate lipid oxidation and regulate vascular structure/vascular cell growth. An important contribution of the endothelium is its role in maintaining vascular tone in large and small arteries by releasing contracting [e.g. endothelin-1 (ET-1)] and relaxing [e.g. nitric oxide (NO), prostacyclin (PGI2)] substances. In health, the primary vasomotor effect of ECs appears to be endothelium-dependent dilation (EDD) signalled by ligands (such as ACh) and intraluminal flow-mediated dilation (FMD) of arteries responding to increased rates of longitudinal blood passage during exercise (Fig. 1C; Hahn & Schwartz 2009). The enhanced relaxation of the surrounding VSM is induced by endothelial generation of signalling molecules such as NO (Fig. 1). Nitric oxide synthases (NOS) produce NO during the catalysis of l-arginine to l-citrulline (see review by McAllister & Laughlin 2006, Fig. 1). Of the three NOS isoforms in mammals (neuronal NOS, nNOS; inducible NOS, iNOS; and endothelial NOS, eNOS), the endothelial form has received the most attention in vascular control for its role in FMD (Dudzinski & Michel 2007). Exposure of ECs to shear stress causes instantaneous NO release partially signalled by phosphorylation of eNOS by AKT (V-AKT murine thymoma viral oncogene homolog 1). NO release so activated is then maintained for a period (Sessa 2005). The diffusion of NO from ECs to the VSM activates cyclic guanosine monophosphate (cGMP) within the VSM cells, which induces vasodilation by pathways that include increased intracellular cGMP, which inhibits calcium entry into the cell and/or activates Ca-ATPase pumps, and increases activation of potassium channels by intracellular cGMP resulting in hyperpolarization and relaxation.
Several vasomotor responses can also be induced by mechanisms of metabolic response (Fig. 1D). For instance, circulating red blood cells that have low oxyhaemoglobin saturation may modulate endothelial NO release by an S-nitrosothiol-based signal, and/or release ATP, inducing a vasodilatory response in a fashion similar to the conducted vasodilation response described previously (Diesen et al. 2008). The action of ATP is believed to be through binding to P2Y purinergic receptors on the luminal surface of the endothelium and/or through degradation of ATP to AMP or adenosine, which may bind to P1 receptors on VSM and initiate the vasodilatory response, or a combination of these mechanisms (Arciero et al. 2008). The skeletal muscle also releases a complement of putative vasodilators during contraction, including potassium, adenosine, hydrogen ions, carbon dioxide and phosphate, although definitive evidence relating these muscle metabolites to vasodilation and the mechanisms involved are scarce (AK Dua et al. 2009). Direct biochemical mechanisms by which the endothelium modulates VSM tone includes the direct release of several endothelium-derived relaxing factors (Fig. 1E); vasodilators such as PGI2, NO and endothelial-derived hyperpolarizing factors (EDHFs) including hydrogen peroxide and potassium ions (Boushel 2003, Bellien et al. 2008) and vasoconstrictor peptides including angiotensin II and ET-1 (Vanhoutte et al. 2005). Excellent recent reviews describing the roles of each of these vasomotor effectors have been published (Félétou & Vanhoutte 2006, Böhm & Pernow 2007, Bian et al. 2008, Rush & Aultman 2008, Wheeler-Jones 2008) and will therefore only be dealt with in this review in respect of exercise responses.
Central control of blood flow
Cardiac output and vascular resistance also adjust to the level of physical activity at a central level, controlled by the autonomic nervous system (e.g. reduction in splanchnic circulation due to sympathetic nervous system control described previously). Neurones involved in the central control of the blood flow include both sympathetic and parasympathetic systems. Parasympathetic control works primarily through controlling heart rate and sympathetic effects influence heart rate, contractility, vascular resistance and venous compliance via barosensitive, thermosensitive and glucosensitive cardiovascular efferent neurones that innervate the vasculature, heart, renal system and the adrenal medulla (Guyenet 2006). When the body is performing maximal exercise, vasodilation within the active muscles is controlled or limited in order to prevent overwhelming of the heart’s pumping capacity, resulting in a drop in arterial pressure (Raven 2008). As described previously, multiple local mechanisms appear to work within the skeletal muscle to increase blood flow and its vasodilator reserve under physical activity, and therefore a separate, central cardiovascular control mechanism is essential for arterial pressure control to prevent hypotension (Delp & O’Leary 2004).
Clifford & Hellsten (2004) concluded that local factors most likely play a stronger role in vasodilation than the autonomic nervous system, although sympathetic vasoconstriction restrains blood flow to active muscle during exercise. It is not known which local factors are indeed responsible for the increase in blood flow to muscle during exercise. Especially problematic is the rapid increase in blood flow that is seen within 1 s of initiation of exercise. Thus, under experimental conditions, the rate of formation and release of the metabolites (vasoactive substances released by active muscle) is not fast enough to account for the near-immediate response of exercise-induced dilation (Kirby et al. 2007). Empirical evidence supports the notion of the existence of a mechanical effect of muscle contraction on initial muscle blood flow (0–5 s) followed by chemical maintenance of this vasodilation (reviewed by Laughlin 1987, Tschakovsky & Sheriff 2004).
Vascular adaptations to chronic physical activity – from genotype to phenotype
The acute effects of physical exercise on the phenotype vascular cells of untrained individuals described in the previous sections is relatively well understood, although not all proposed mechanisms are agreed upon. It has been known for some time that chronic exposure to physical activity (i.e. exercise training) results in improved cardiovascular function as seen in increased maximal oxygen consumption, increased maximal cardiac output and increased blood flow capacity in skeletal and cardiac muscle. In relation to the prevention and treatment of many chronic diseases, including cardiovascular disease, the molecular pathways that are modified by exercise training are just being revealed. The consideration of how repetitive (ideally daily) exposure to the demands of exercise is responsible for the improved cardiovascular system function during exercise in trained individuals leads to interesting questions regarding the long-term effects of altered gene expression under recurring, sustained physical activity. Long-term adaptation to resistance training is probably due to the cumulative molecular effects of each exercise session (Zanchi et al. 2009). The effects of sustained efforts on regulation of DNA translation, mRNA transcription, and protein expression and activity can yield a new, stable phenotype referred to as ‘training adaptation’ (Hansen et al. 2005). Although such effects can influence all organ systems in the body, our focus in this review is on established changes from exercise to the vasculature at the genomic level that ultimately lead to enduring changes in vascular cell phenotype and blood vessel physiology.
Vascular gene adaptations to mechanical stressors in exercise
In reference to the question of what signals generated by exercise bouts can serve as modifiers of EC gene expression and phenotype, at this time the most likely candidates seem to be: chemical signals (e.g. cytokines, metabolites released from muscle, neurone-humoral factors) and haemodynamic mechanical signals acting on the endothelium (e.g. shear stress, blood pressure and stretch; circumferential stress). There is a growing body of evidence that blood flow-mediated changes, due to repeated exercise that impose shear stress and/or stretch on the artery wall, can initiate a cascade of altered gene expression in arterial ECs. Indeed, some exercise physiologists consider that sufficient exposure to these signals induced by exercise bouts may be required to maintain a ‘normal’ human EC phenotype (Booth & Roberts 2008, Laughlin et al. 2008). There indeed is evidence that the endothelial phenotype of a sedentary individual is actually a deviation from what we should consider the true benchmark. This normal or healthy endothelial phenotype is crucial to the maintenance of normal vascular tone, in preventing VSM proliferation and migration like that seen in atherosclerosis, in blunting inflammation, innate immunity, fibrinolysis and the prothrombosis-anticoagulation equilibrium (Mensah 2007). It has been known for over a decade that a common denominator in blood vessel development (neovascularization) is increased blood flow and consequent shear stress and wall tension and it appears these haemodynamic signals participate in the development of vascular cell phenotypes required for normal vascularization to occur (Hudlicka 1998). Data from both animal and human training studies indicate that a major factor in endothelial adaptation to long-term exercise appears to be vessel size and tissue studied. Under long-term training, resistance vessels and the aorta of the heart have enhanced EDD, but this chronic training does not have a generalized effect on endothelial function in conduit arteries of non-muscular vascular beds (reviewed in Jasperse & Laughlin 2006). For muscular vascular beds, there is increased shear stress during training bouts with long-term training, and arterial remodelling is observed in predominantly trained muscles, with subsequently improved FMD of large conduit arteries and superior vasodilatory capacity during isolated exercise (Walther et al. 2008). This study also demonstrated similar effects in conduit and resistance vessels of the upper limb after long-term intensive leg (lower limb) training. The length of exercise training appears to be the critical factor in determining which vessels are affected, likely due to the localized initial response in gene upregulation (mRNA) in the resistance vessel endothelium under acute bouts of training, followed by structural adaptations (e.g. protein expression, vascular remodelling) induced over longer periods of sustained training (Jasperse & Laughlin 2006, Tanaka et al. 2006).
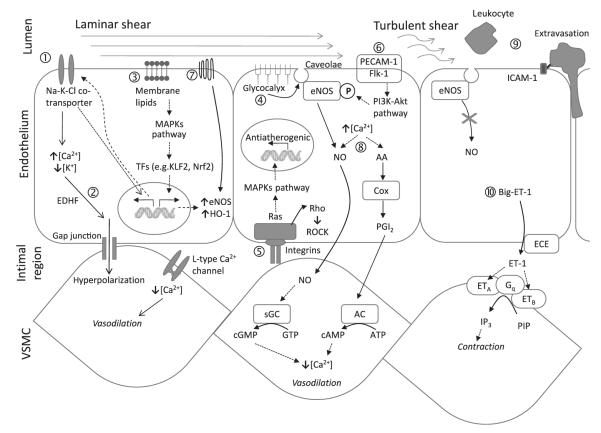
Chronic exercise has been demonstrated to modify the expression of many EC gene products via mechanotransduction. Mechanotransduction by shear stress and the biochemical pathways influenced downstream have been the topic of a number of excellent reviews (Wamhoff et al. 2006, Chien 2008, Chiu et al. 2009, Hahn & Schwartz 2009, Pan 2009). The molecular events specifically related to exercised-induced blood flow in the endothelium and surrounding VSM are presented as a schematic representation in Figure 2. The increased shear at the endothelial surface due to physical activity can result in distortion of the cell monolayer. Steady laminar, but not oscillatory, flow can markedly increase Na-K-Cl co-transporter mRNA and protein in cultured ECs, and also increased K+ and Cl− channel conductances (Suvatne et al. 2001). Flows of 19 dyn cm−2 for periods of 6, 12, 24 and 48 h resulted in progressive cellular elongation and alignment in the direction of flow, likely due to cytoskeletal reorganization. The authors propose that the co-transporter protein may act as a flow-sensor itself, upregulating transcription of co-transporter protein components (Fig. 2①). The additional co-transporter can lead to EDHF-type mediation, evoked by an increase in endothelial [Ca2+], activating endothelial KCa channels to elicit hyperpolarization conducted via myoendothelial gap junctions to the smooth muscle, resulting in decreases in [Ca2+] and consequently vasodilation (Ungvari et al. 2002) (Fig. 2①).
Figure 2.
Cellular and molecular effects of exercise-induced shear stress. Under conditions of prolonged laminar flow ① increase in Na-K-Cl co-transporter expression leads to ② EDHF-type-mediated vasodilation. ③ Membrane lipids as mechanotransducers. ④ Glycocalyx-mediated stimulation of caveolae-bound eNOS, producing NO. ⑤ Integrin phosphorylation and activation of non-receptor tyrosine kinase cascade. ⑥ Phosphoinositide-3-kinase (PI3K)-dependent pathways for phosphorylation of eNOS. ⑦ Flow-mediated activation of heterotrimeric G proteins. ⑧ Release of NO and PGI2 as vasodilators and anti-platelet mediators. ⑨ Leucocyte recruitment and binding to the endothelium in areas of low or turbulent shear stress. ⑨ Expression of vasoconstrictor endothelin-1 (ET-1) under conditions of reduced or turbulent flow. AA, arachidonic acid; Cox, cyclooxygenase; eNOS, endothelial nitric oxide synthase; ECE, endothelin-converting enzyme; EDHF, endothelial-derived hyperpolarizing factor;  , gene transcription; ICAM1, intercellular adhesion molecule 1; KLF2, Krüppel-like factor 2; MAPK, mitogen-activated protein kinase; Nrf2, nuclear factor erythroid 2-like 2; PGI2, prostacyclin; sGC, soluble guanylate cyclase; TFs, transcription factors.
, gene transcription; ICAM1, intercellular adhesion molecule 1; KLF2, Krüppel-like factor 2; MAPK, mitogen-activated protein kinase; Nrf2, nuclear factor erythroid 2-like 2; PGI2, prostacyclin; sGC, soluble guanylate cyclase; TFs, transcription factors.
Steady rhythmic flow, as is observed when exercising, also has impacts on the endothelial lipid bilayer, increasing membrane fluidity (Butler et al. 2002). Acting as a transducer, lateral movements of lipid components of the membrane can discriminate between different temporal shear gradients to initiate the events leading to mitogen-activated protein kinase (MAPK) activation in the cell (Fig. 2③). Prolonged laminar shear stress induces anticoagulant and anti-inflammatory gene expression through the activation of the transcription factors including Krüppel-like factor 2 and nuclear factor erythroid 2-like 2, including upregulation of the antioxidant enzyme haem oxygenase 1 and also eNOS (Boon & Horrevoets 2009). Other membrane components, such as the glycocalyx, may play a role in mechanotransduction due to their intimate association with the membrane bilayer (Fig. 2④). A possible scenario is that, at least in part, shear-induced NO production is mediated by eNOS in caveolae that may be stimulated through the solid matrix components of the glycocalyx (Tarbell & Pahakis 2006).
Exercise conditions produce laminar, yet pulsatile blood flow that regulates the orientation of ECs lining blood vessels and influences critical processes such as angiogenesis (Thodeti et al. 2009). These shear stress signals are transmitted through the cytoskeleton to the intimal region at the basal endothelial surface (Fig. 2⑤). This triggers integrins to phosphorylate and activate a multiple complex of non-receptor tyrosine kinases (FAK, c-Src, Shc, paxillin and p130CAS), adaptor proteins (Grb2, Crk) and guanine nucleotide exchange factors (Sos, C3G), thereby activating Ras family GTPase (reviewed in Chatzizisis et al. 2007). Active Ras activates various parallel downstream cascades of serine kinases in a phosphorylation ‘chain reaction’ ultimately activating MAPKs. These molecular mechanisms result in shear stress-induced upregulation of genes mediating anti-atherogenic effects by promoting anti-apoptotic and anti-proliferative signals, by increasing vascular NO bioavailability and by vascular remodelling (Kojda & Hambrecht 2005). In contrast, in regions with low and disturbed flow (either due to vessel shape or sedentary lifestyle), the atheroprotective genes are suppressed, whereas the pro-atherogenic genes are upregulated (e.g. c-Jun NH2-terminal kinases), thereby promoting the atherosclerotic process (Hahn et al. 2009). A complex consisting of platelet EC adhesion molecule-1 and Flk-1 are also activated by shear stress, respectively (Abumiya et al. 2002), and initiate phosphoinositide-3-kinase (PI3K)-dependent phosphorylation pathways (Fig. 2⑥). Kinase Akt (or protein kinase B) is a cytosolic protein that is regulated by PI3K (Hahn & Schwartz 2009). This pathway is an important determinant in activating eNOS phosphorylation at Ser 1177 for proper NO production.
An early flow-mediated response in ECs is the rapid activation of heterotrimeric G proteins (Fig. 2⑦) and this may clarify the reaction of the endothelium to different flow profiles under exercise and rest conditions (White & Frangos 2007). Highlighting this difference, in cultured ECs, a sudden temporal onset of flow causes a rapid rise in NO production, a process that is both calcium and G protein dependent (Kuchan et al. 1994). However, if the shear stress is prolonged, a sustained release of NO occurs that is both calcium and G protein independent. It is critical, therefore, to understand both the change in flow and the rate-of-change in flow in order to understand the influence of exercise on biochemical mechanisms of vasomotor control.
In vessel areas of smooth, laminar blood flow, shear stresses are maximal along the vessel wall, and shear stress detectors trigger the secretion and release of endothelial vasodilators such as NO and PGI2 and protectors such as tissue plasminogen activator (t-PA; Lowe 2003), a serine protease that catalyses the conversion of plasminogen to plasmin, the major enzyme responsible for clot breakdown. Such streamlined flow would occur under exercise training, allowing t-PA to restrict platelet adhesion and aggregation as well as fibrin formation to sites of endothelial injury. A 3-month aerobic exercise programme resulted in a significant increase in t-PA release capacity in human subjects who were overweight or obese, elevating them to levels comparable to their normal weight peers (Van Guilder et al. 2005). Deficiencies in t-PA mice demonstrate accelerated atherosclerotic fibrin deposition (Carmeliet et al. 1994) and in humans, reduced endothelial t-PA release rates have been linked to increased atheromatous plaques (Hoffmeister et al. 1998). Highlighting the variable and complex nature of gene regulation under different shear stress conditions and model systems, Ulfhammer et al. (2009) reported that high laminar shear stress in cultured ECs suppresses t-PA expression in a time- and magnitude-dependent manner. The authors suggested that this may contribute to the increased risk of atherothrombotic events in hypertension, although it is important to be careful when extrapolating conclusions from tissue culture to in vivo systems.
The vasodilation and anti-platelet mediator activity of NO and PGI2 in response to exercise have been examined extensively (Fig. 2⑧). It has been shown that both NO and PGI2, a cyclooxygenase-derived relaxing factor, inhibit activation of platelets and regulate vasomotor actions (Hermann 2006). Reduced NO and PGI2 levels can result in endothelial dysfunction, which is recognized as the first step in the atherogenic process. Production of NO by the endothelium, which is signalled by increased flow over these cells, can influence expression of antioxidant enzymes and cellular inflammation in vascular tissue (Harrison et al. 2006). In contrast, mice in a state of forced physical inactivity (designed to mimic a sedentary lifestyle) had a strong reduction of vascular eNOS gene expression accompanied by the development of endothelial dysfunction (Suvorava et al. 2004). It has been suggested that one mechanosensor for NO production is the endothelial glycocalyx (Tarbell & Pahakis 2006). Addition of enzymes that degrade specific endothelial glycosaminoglycan components revealed that the glycocalyx mediates the shear-induced production of NO, but not PGI2 (Tarbell & Ebong 2008). These experiments reinforce the concept of redundant, but mechanistically separate modes of vasomotor control in the endothelium.
Mechanisms of atherosclerosis in the absence of exercise
Physical activity results in high velocity, rhythmic blood flow that induces anti-atherogenic molecular pathways and expression of protective gene families. A sedentary lifestyle results in disturbed vascular flow (i.e. nonuniform and irregular flow and recirculation). The influence of blood flow in atherosclerosis is can be inferred from the presence of vascular inflammation and plaques are distributed at lesser curvature of bends and near side branches, where blood flow rates are relatively low (Ridger et al. 2008). A key initial step in this process involves the recruitment and binding of leucocytes to the endothelium of the artery (Fig. 2⑨).
In general, leucocytes move quickly along vessels without adhering to the walls. However, in the presence of adhesion molecules, they can become ‘tethered’ through interaction with P- or L-selectin on the microvilli of leucocytes and on the endothelium (reviewed by Ley et al. 2007). Insufficient shear stress due to reduced flow or disturbed flow in arteries, which appears to be more associated with sedentary behaviour, not only negates the beneficial effects of training on the endothelium but also upregulates the expression of leucocyte adhesion receptors, such as intercellular adhesion molecule 1 and vascular cell-adhesion molecule 1, and chemokines, such as monocyte chemotactic protein 1 under conditions of disturbed flow (Chen et al. 2001). These factors also appear early in the development of atherosclerosis. It is believed that together, these receptors/signalling molecules produced by ECs initiate and maintain inflammation within the vessel wall leading to development of vascular disease. These areas of low or disturbed flow occur preferentially at arterial bifurcations and bends, and are more susceptible to atherogenesis due to the low-flow, low-shear recirculation of blood cells and proteins in contract with the vessel wall (Lowe 2003). It is not the low-flow as such that causes the atherogenic response. Rather, systemic inflammation related markers (e.g. IL-6, IL-8 and TNF-α) can act as exogenous stimuli to induce the expression of adhesion molecules by activating ECs (Elenkov 2008). Under conditions of low-flow, circulating leucocytes can adhere to the EC surface, and transmigrate across the endothelial lining (Fig. 2⑨), initiating the development of an atherosclerotic lesion (Augustin et al. 2009). In addition, the highly potent vasoconstrictor ET-1 is also expressed under conditions of reduced or turbulent flow. ET-1 is generated from big ET-1 by endothelin-converting enzyme (ECE)-1 in ECs and secreted predominantly towards underlying VSM cells. ET-1 binds to smooth muscle endothelin receptors (Fig. 2⑨; subtypes ETA and ETB). These receptors are coupled to a Gq-protein and upon binding, ET-1 triggers the formation of inositol triphosphate (IP3), which causes the release of calcium in the smooth muscle and subsequent contraction and vasoconstriction (Remuzzi et al. 2002).
Conclusions
Exercise training and regular physical activity have a strong positive link with vascular function and exercise training can modify the vascular structure and the function of vascular cells. One important component of these effects of exercise training is a greatly improved endothelium-dependent vasodilation that is believed to be the result of increased shear stress over the endothelium during exercise training bouts. A number of mechanosensory mechanisms have been elucidated that may contribute to the effects of exercise on vascular function, but there are still many unanswered questions regarding the interactions among molecular pathways and yet undiscovered players in the control of vascular tone. For instance, the increase in flow brought on by regular exercise can reduce circulating levels of viscosity and haemostatic and inflammatory variables that may interact through increased laminar shear stress, releasing vasoactive substances such as NO and PGI2, decreasing permeability to plasma lipoproteins as well as the adhesion of leucocytes (Pan 2009). However, the optimal rate-of-flow and rate-of-change in flow are important factors in determining whether anti-atherogenic or atherogenic processes proceed. In addition, many haemodynamic variables depend on the vessel size and tissue type in which the blood vessels are located. As significant as these hurdles to understanding the mechanism responsible for exercise-induced alterations in vascular cell gene expression are, they in no way undermine the established benefits of regular physical activity to the cardiovascular system and to general overall health. Aspects of maintaining the cardiovascular system and controlling vascular cell gene expression – key components of health – are just now beginning to be understood. The future of improving this understanding is bright at this time.
Footnotes
Conflict of interest There is no conflict of interest.
References
- Abumiya T, Sasaguri T, Taba Y, Miwa Y, Miyagi M. Shear stress induces expression of vascular endothelial growth factor receptor Flk-1/KDR through the CT-rich Sp1 binding site. Arterioscler Thromb Vasc Biol. 2002;22:907–913. doi: 10.1161/01.atv.0000018300.43492.83. [DOI] [PubMed] [Google Scholar]
- Arciero JC, Carlson BE, Secomb TW. Theoretical model of metabolic blood flow regulation: roles of ATP release by red blood cells and conducted responses. Am J Physiol Heart Circ Physiol. 2008;295:H1562–H1571. doi: 10.1152/ajpheart.00261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Beaglehole R, Ebrahim S, Reddy S, Voûte J, Leeder S. Prevention of chronic diseases: a call to action. Lancet. 2007;370:2152–2157. doi: 10.1016/S0140-6736(07)61700-0. [DOI] [PubMed] [Google Scholar]
- Bellien J, Thuillez C, Joannides R. Contribution of endothelium-derived hyperpolarizing factors to the regulation of vascular tone in humans. Fundam Clin Pharmacol. 2008;22:363–377. doi: 10.1111/j.1472-8206.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- Bian K, Doursout M, Murad F. Vascular system: role of nitric oxide in cardiovascular diseases. J Clin Hypertens. 2008;10:304–310. doi: 10.1111/j.1751-7176.2008.06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76:8–18. doi: 10.1016/j.cardiores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Boon RA, Horrevoets AJG. Key transcriptional regulators of the vasoprotective effects of shear stress. Hämostaseologie. 2009;29:39–40. 41–43. [PubMed] [Google Scholar]
- Booth FW, Roberts CK. Linking performance and chronic disease risk: indices of physical performance are surrogates for health. Br J Sports Med. 2008;42:950–952. doi: 10.1136/bjsm.2008.052589. [DOI] [PubMed] [Google Scholar]
- Boushel R. Metabolic control of muscle blood flow during exercise in humans. Can J Appl Physiol. 2003;28:754–773. doi: 10.1139/h03-057. [DOI] [PubMed] [Google Scholar]
- Butler PJ, Tsou T, Li JY, Usami S, Chien S. Rate sensitivity of shear-induced changes in the lateral diffusion of endothelial cell membrane lipids: a role for membrane perturbation in shear-induced MAPK activation. FASEB J. 2002;16:216–218. doi: 10.1096/fj.01-0434fje. [DOI] [PubMed] [Google Scholar]
- Carlson B, Arciero J, Secomb T. Theoretical model of blood flow autoregulation: roles of myogenic, shear-dependent, and metabolic responses. Am J Physiol Heart Circ Physiol. 2008;295:H1572–H1579. doi: 10.1152/ajpheart.00262.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, de Vos R, van den Oord JJ, Collen D, Mulligan RC. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen H, Liu X, Lei S, Mao Y, Zhang W. Effects of flow shear stress on the expression of adhesion molecules of endothelial cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2001;18:201–205. [PubMed] [Google Scholar]
- Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36:554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu J, Usami S, Chien S. Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Ann Med. 2009;41:19–28. doi: 10.1080/07853890802186921. [DOI] [PubMed] [Google Scholar]
- Clifford PS. Skeletal muscle vasodilatation at the onset of exercise. J Physiol. 2007;583:825–833. doi: 10.1113/jphysiol.2007.135673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand. 1998;162:411–419. doi: 10.1046/j.1365-201X.1998.0324e.x. [DOI] [PubMed] [Google Scholar]
- Delp MD, O’Leary DS. Integrative control of the skeletal muscle microcirculation in the maintenance of arterial pressure during exercise. J Appl Physiol. 2004;97:1112–1118. doi: 10.1152/japplphysiol.00147.2003. [DOI] [PubMed] [Google Scholar]
- Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells: evidence for an s-nitrosothiol-based signal. Circ Res. 2008;103:545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J Physiol. 2007;579:175–186. doi: 10.1113/jphysiol.2006.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua AK, Dua N, Murrant CL. Skeletal muscle contraction-induced vasodilator complement production is dependent on stimulus and contraction frequency. Am J Physiol Heart Circ Physiol. 2009;297:H433–H442. doi: 10.1152/ajpheart.00216.2009. [DOI] [PubMed] [Google Scholar]
- Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52:40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Engel G, Froelicher VF. ECG exercise testing. In: Fuster V, O’Rourke RA, Walsh RA, Poole-Wilson P, editors. Hurst’s The Heart. 2008. http://www.accessmedicine.com/content.aspx?aID=3049923. [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Govindaraju DR, Cupples LA, Kannel WB, O’Donnell CJ, Atwood LD, D’Agostino RB, Fox CS, Larson M, Levy D, Murabito J, Vasan RS, Splansky GL, Wolf PA, Benjamin EJ. Genetics of the Framingham Heart Study population. Adv Genet. 2008;62:33–65. doi: 10.1016/S0065-2660(08)00602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau AJ, Barth C, Geletneky B, Ling P, Palm F, Lichy C, Becher H, Buggle F. Association between recent sports activity, sports activity in young adulthood, and stroke. Stroke. 2009;40:426–431. doi: 10.1161/STROKEAHA.108.527978. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C, Orr AW, Sanders JM, Jhaveri KA, Schwartz MA. The subendothelial extracellular matrix modulates JNK activation by flow. Circ Res. 2009;104:995–1003. doi: 10.1161/CIRCRESAHA.108.186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AK, Fischer CP, Plomgaard P, Andersen JL, Saltin B, Pedersen BK. Skeletal muscle adaptation: training twice every second day vs. training once daily. J Appl Physiol. 2005;98:93–99. doi: 10.1152/japplphysiol.00163.2004. [DOI] [PubMed] [Google Scholar]
- Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction nitric oxide and vascular inflammation. J Intern Med. 2006;259:351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Krustrup P, Iaia FM, Secher NH, Bangsbo J. Partial neuromuscular blockade in humans enhances muscle blood flow during exercise independently of muscle oxygen uptake and acetylcholine receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1106–R1112. doi: 10.1152/ajpregu.90477.2008. [DOI] [PubMed] [Google Scholar]
- Hermann M. Cyclooxygenase-2 and nitric oxide. J Cardiovasc Pharmacol. 2006;47(Suppl. 1):S21–S25. doi: 10.1097/00005344-200605001-00005. [DOI] [PubMed] [Google Scholar]
- Hoffmeister HM, Jur M, Ruf-Lehmann M, Helber U, Heller W, Seipel L. Endothelial tissue-type plasminogen activator release in coronary heart disease: transient reduction in endothelial fibrinolytic reserve in patients with unstable angina pectoris or acute myocardial infarction. J Am Coll Cardiol. 1998;31:547–551. doi: 10.1016/s0735-1097(97)00531-7. [DOI] [PubMed] [Google Scholar]
- Hudlicka O. Is physiological angiogenesis in skeletal muscle regulated by changes in microcirculation? Microcirculation. 1998;5:7–23. [PubMed] [Google Scholar]
- Jasperse JL, Laughlin MH. Endothelial function and exercise training: evidence from studies using animal models. Med Sci Sports Exerc. 2006;38:445–454. doi: 10.1249/01.mss.0000191187.24525.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol. 2007;583:861–874. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res. 2005;67:187–197. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Kuchan MJ, Jo H, Frangos JA. Role of G proteins in shear stress-mediated nitric oxide production by endothelial cells. Am J Physiol. 1994;267:C753–C758. doi: 10.1152/ajpcell.1994.267.3.C753. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol. 1987;253:H993–H1004. doi: 10.1152/ajpheart.1987.253.5.H993. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Cardiovascular response to exercise. Am J Physiol. 1999;277:S244–S259. doi: 10.1152/advances.1999.277.6.S244. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Roseguini B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with interval sprint training versus aerobic endurance training. J Physiol Pharmacol. 2008;59(Suppl. 7):71–88. [PMC free article] [PubMed] [Google Scholar]
- Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol. 2008;104:588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Lowe GDO. Virchow’s triad revisited: abnormal flow. Pathophysiol Haemost Thromb. 2003;33:455–457. doi: 10.1159/000083845. [DOI] [PubMed] [Google Scholar]
- McAllister RM, Laughlin MH. Vascular nitric oxide: effects of physical activity, importance for health. Essays Biochem. 2006;42:119–131. doi: 10.1042/bse0420119. [DOI] [PubMed] [Google Scholar]
- Mensah GA. Healthy endothelium: the scientific basis for cardiovascular health promotion and chronic disease prevention. Vascul Pharmacol. 2007;46:310–314. doi: 10.1016/j.vph.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Murrant CL, Sarelius IH. Coupling of muscle metabolism and muscle blood flow in capillary units during contraction. Acta Physiol Scand. 2000;168:531–541. doi: 10.1046/j.1365-201x.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- Osada T, Katsumura T, Hamaoka T, Inoue S, Esaki K, Sakamoto A, Murase N, Kajiyama J, Shimomitsu T, Iwane H. Reduced blood flow in abdominal viscera measured by Doppler ultrasound during one-legged knee extension. J Appl Physiol. 1999;86:709–719. doi: 10.1152/jappl.1999.86.2.709. [DOI] [PubMed] [Google Scholar]
- Pan S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid Redox Signal. 2009;11:1669–1682. doi: 10.1089/ars.2009.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowicz R, Wolszakiewicz J. Cardiac rehabilitation following myocardial infarction. Cardiol J. 2008;15:481–487. [PubMed] [Google Scholar]
- Raven PB. Recent advances in baroreflex control of blood pressure during exercise in humans: an overview. Med Sci Sports Exerc. 2008;40:2033–2036. doi: 10.1249/MSS.0b013e318180bc41. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Perico N, Benigni A. New therapeutics that antagonize endothelin: promises and frustrations. Nat Rev Drug Discov. 2002;1:986–1001. doi: 10.1038/nrd962. [DOI] [PubMed] [Google Scholar]
- Ridger V, Krams R, Carpi A, Evans PC. Hemodynamic parameters regulating vascular inflammation and atherosclerosis: a brief update. Biomed Pharmacother. 2008;62:536–540. doi: 10.1016/j.biopha.2008.07.053. [DOI] [PubMed] [Google Scholar]
- Röckl KSC, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life. 2008;60:145–153. doi: 10.1002/iub.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush JWE, Aultman CD. Vascular biology of angiotensin and the impact of physical activity. Appl Physiol Nutr Metab. 2008;33:162–172. doi: 10.1139/H07-147. [DOI] [PubMed] [Google Scholar]
- Sessa WC. Regulation of endothelial derived nitric oxide in health and disease. Mem Inst Oswaldo Cruz. 2005;100(Suppl. 1):15–18. doi: 10.1590/s0074-02762005000900004. [DOI] [PubMed] [Google Scholar]
- Suvatne J, Barakat AI, O’ Donnell ME. Flow-induced expression of endothelial Na-K-Cl cotransport: dependence on K(+) and Cl(−) channels. Am J Physiol Cell Physiol. 2001;280:C216–C227. doi: 10.1152/ajpcell.2001.280.1.C216. [DOI] [PubMed] [Google Scholar]
- Suvorava T, Lauer N, Kojda G. Physical inactivity causes endothelial dysfunction in healthy young mice. JAm Coll Cardiol. 2004;44:1320–1327. doi: 10.1016/j.jacc.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Shimizu S, Ohmori F, Muraoka Y, Kumagai M, Yoshizawa M, Kagaya A. Increases in blood flow and shear stress to nonworking limbs during incremental exercise. Med Sci Sports Exerc. 2006;38:81–85. doi: 10.1249/01.mss.0000191166.81789.de. [DOI] [PubMed] [Google Scholar]
- Tarbell JM, Ebong EE. The endothelial glycocalyx: a mechano-sensor and transducer. Sci Signal. 2008;1(Pt 8) doi: 10.1126/scisignal.140pt8. http://sciencesignaling.org/cgi/content/abstract/sigtrans;1/40/pt8. [DOI] [PubMed] [Google Scholar]
- Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259:339–350. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104:1123–1130. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol. 2004;97:731–738. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol. 2004;97:739–747. doi: 10.1152/japplphysiol.00185.2004. [DOI] [PubMed] [Google Scholar]
- Ulfhammer E, Carlström M, Bergh N, Larsson P, Karlsson L, Jern S. Suppression of endothelial t-PA expression by prolonged high laminar shear stress. Biochem Biophys Res Commun. 2009;379:532–536. doi: 10.1016/j.bbrc.2008.12.105. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Koller A. Increases in endothelial Ca(2+) activate K(Ca) channels and elicit EDHF-type arteriolar dilation via gap junctions. Am J Physiol Heart Circ Physiol. 2002;282:H1760–H1767. doi: 10.1152/ajpheart.00676.2001. [DOI] [PubMed] [Google Scholar]
- Van Guilder GP, Hoetzer GL, Smith DT, Irmiger HM, Greiner JJ, Stauffer BL, Desouza CA. Endothelial t-PA release is impaired in overweight and obese adults but can be improved with regular aerobic exercise. Am J Physiol Endocrinol Metab. 2005;289:E807–E813. doi: 10.1152/ajpendo.00072.2005. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Félétou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. J Am Soc Hypertens. 2009;22:250–256. doi: 10.1038/ajh.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther G, Nottin S, Karpoff L, Pérez-Martin A, Dauzat M, Obert P. Flow-mediated dilation and exercise-induced hyperaemia in highly trained athletes: comparison of the upper and lower limb vasculature. Acta Physiol (Oxf) 2008;193:139–150. doi: 10.1111/j.1748-1716.2008.01834.x. [DOI] [PubMed] [Google Scholar]
- Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;98:868–878. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang W, Li R, Li Y, Tian G, Goodman L, Fan W, Zhang J, Li J, Zhang J, et al. The diploid genome sequence of an Asian individual. Nature. 2008;456:60–65. doi: 10.1038/nature07484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DA, Srinivasan M, Egholm M, Shen Y, Chen L, McGuire A, He W, Chen Y, Makhijani V, Roth GT, et al. The complete genome of an individual by massively parallel DNA sequencing. Nature. 2008;452:872–876. doi: 10.1038/nature06884. [DOI] [PubMed] [Google Scholar]
- Wheeler-Jones CPD. Regulation of endothelial prostacyclin synthesis by protease-activated receptors: mechanisms and significance. Pharmacol Rep. 2008;60:109–118. [PubMed] [Google Scholar]
- White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro Y, Duling BR. Participation of intracellular Ca2+ stores in arteriolar conducted responses. Am J Physiol Heart Circ Physiol. 2003;285:H65–H73. doi: 10.1152/ajpheart.00662.2002. [DOI] [PubMed] [Google Scholar]
- Zanchi N, de Siqueira Filho M, Lira F, Rosa J, Yamashita A, de Oliveira Carvalho C, Seelaender M, Lancha A., Jr. Chronic resistance training decreases MuRF-1 and Atrogin-1 gene expression but does not modify Akt, GSK-3beta and p70S6K levels in rats. Eur J Appl Physiol. 2009;106:415–423. doi: 10.1007/s00421-009-1033-6. [DOI] [PubMed] [Google Scholar]