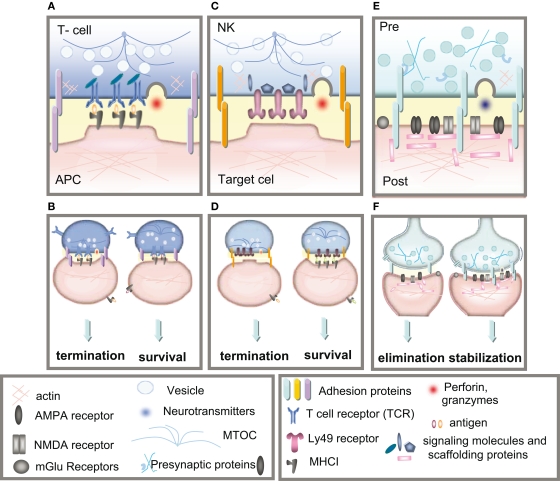
Figure 2.
Immune and neuronal synapses. Highly simplified schematics of these synapses illustrate the common core components of these asymmetric junctions. (A) A cytotoxic T-cell immune synapse is formed between an antigen-specific T-cell and a cell infected with an intracellular pathogen. The close junction is formed from a ring of adhesion proteins (purple cylinders) surrounding an inner signaling molecular domain of antigen receptors (MHCI; gray y) bound to T-cell receptors (TCR; blue Y). Cytokine receptors also cluster in the synapse (not shown in the diagram) where they are exposed to cytokines secreted into the synapse. (B, left) Activation of TCR signaling by MHCI molecules presenting non-self antigens (orange ovals) causes a signaling cascade resulting in polarization of the actin and microtubule cytoskeleton (blue lines) in the T-cell, recruitment of lytic granules (circles) and cell-surface receptors and co-stimulatory molecules (including trans-synaptic adhesion molecules [purple ovals]) to the synapse, secretion of lytic granules (red) and apoptosis of the infected cell. (B, right) Conversely, TCR interaction with an MHC1 molecule containing a non-TCR specific peptide (orange ovals) does not affect microtubule reorganization and does not recruit lytic granules, resulting in termination of the contact and survival the APC. (C)The lytic natural killer (NK) cell immune synapse is also specialized for mediating cytotoxicity. An encounter between an NK cell and a target cell results in adhesion (orange ovals). The balance between activating and inhibitory receptor (pink) signaling at the cell–cell contact determines the outcome of the interaction. (D, left) A lack of MHCI on the target cell, caused by viral infection or tumorigenesis, triggers a signaling cascade in the NK cell resulting in reorganization of the actin cytoskeleton, clustering of cell-surface receptors (pink) and signaling molecules (blue) in the NK cell, recruitment and secretion of lytic granules, ultimately resulting in lysis of the target cell. (D, right) Conversely, the presence of MHCI (gray y) on the target cell results in binding of MHCI to NK inhibitory receptors, including PirB and Ly49s and initiates dominant inhibitory signaling, preventing the formation of the NK cell activation synapse and resulting in survival of the target cell. (E) Glutamatergic synapses in the mammalian CNS are comprised of several major protein classes. In the presynaptic axon terminal (blue), synaptic vesicles (circles) containing the neurotransmitter glutamate (blue) cycle at the active zone, which is composed of many kinds of proteins including presynaptic scaffolding proteins (blue Xs and hooks). The presynaptic terminal is separated from the postsynaptic dendrite (pink) by the synaptic cleft (yellow). A number of families of trans-synaptic adhesion molecules (blue ovals) span this cleft, providing a molecular connection capable of rapid signaling between the pre- and postsynaptic membranes. Glutamate receptors (gray), including AMPA and NMDA receptors, are found in the postsynaptic membrane, where they are associated with a large number of scaffolding and signaling proteins (pink rectangles) that together comprise the postsynaptic density. (F) If the synapse is weakened by long-term depression (LTD) then it can be eliminated (left), but if it is strengthened by long-term potentiation (LTP) then it will be stabilized and grow (right).