Abstract
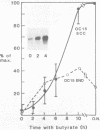
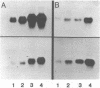
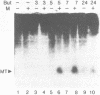
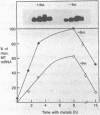
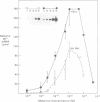
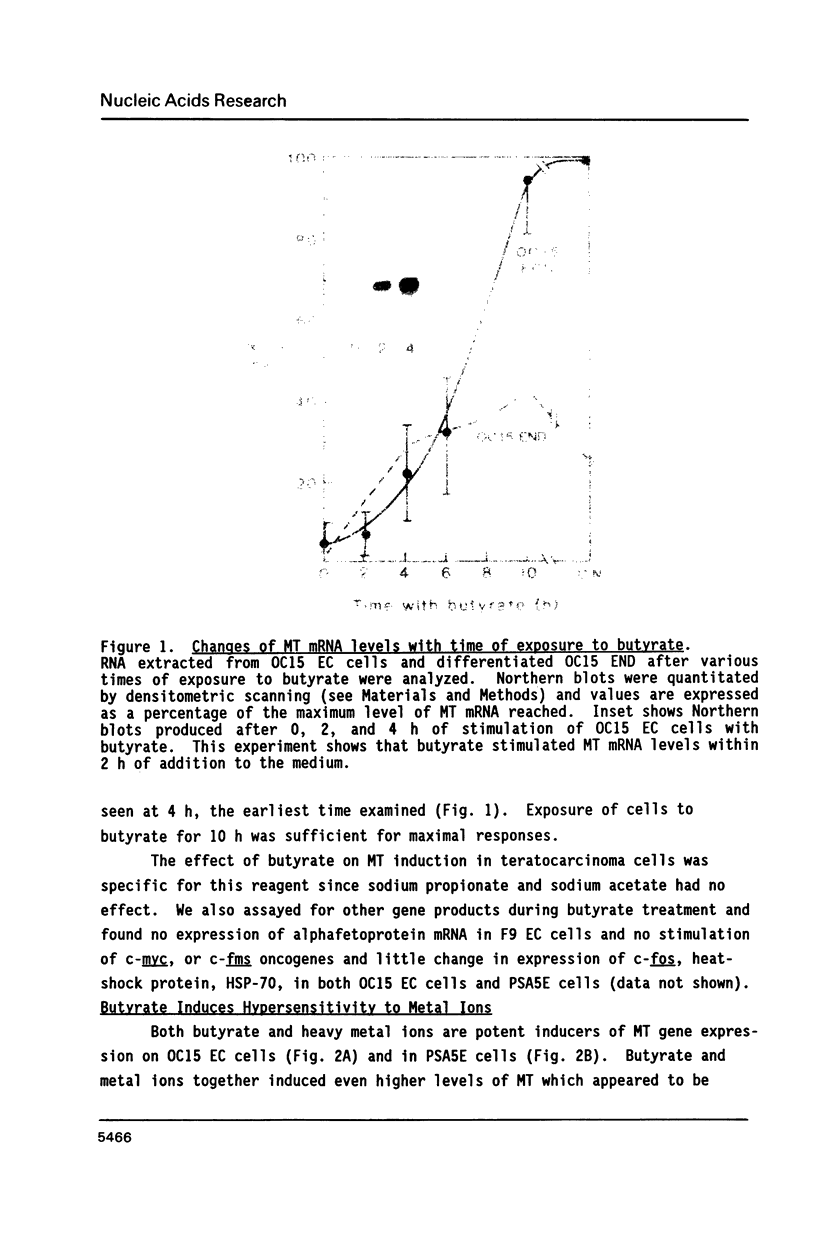
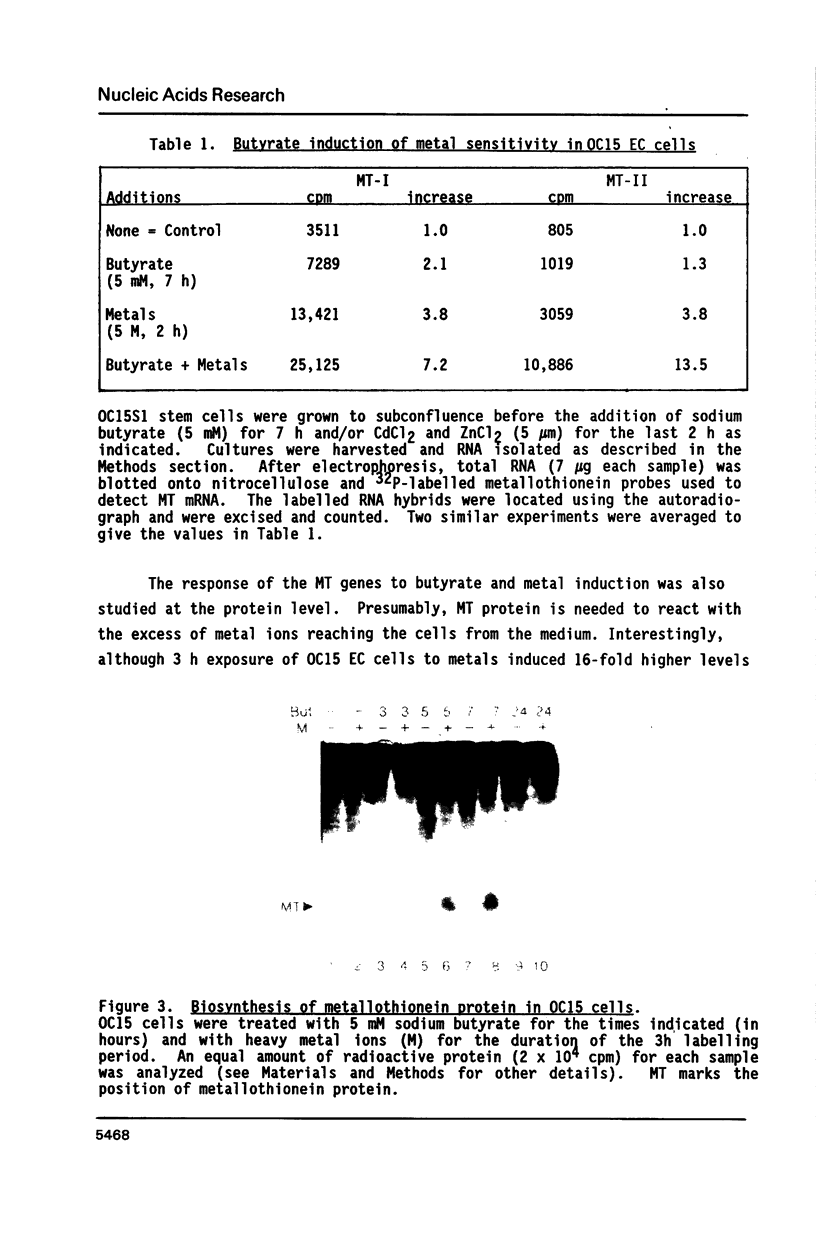
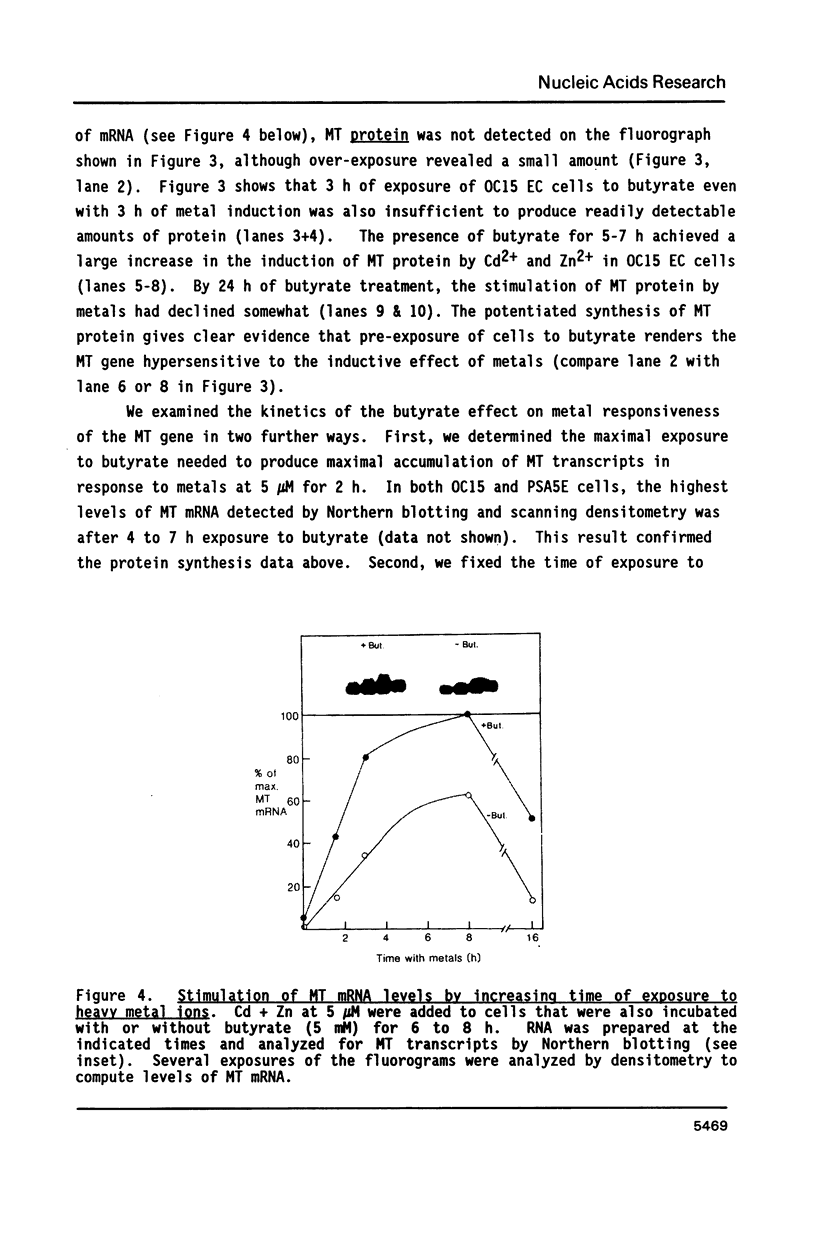
The expression of metallothionein genes (MT-I and MT-II) was shown to be enhanced within 2 h of addition of 2.5-5 mM sodium butyrate to cultures of teratocarcinoma cells. Both undifferentiated stem cells (F9 and OC15) and differentiated cells (PSA5E and OC15 END) reacted similarly to butyrate by increased accumulation of MT mRNAs. As expected, all of the teratocarcinoma cells that were tested also responded to Zn2+ and Cd2+ by 5- to 10-fold increases in MT mRNA accumulation within 2-24 h of metal addition to the culture media. Surprisingly, MT genes in cells pretreated with butyrate were hypersensitive to metal induction, and this was demonstrated by accumulated transcript levels and by synthesis of MT protein. The maximal metal response was obtained by exposure of cells to butyrate for around 5-8 h together with 10 microM heavy metals. Metal additions to culture media over a range of concentrations and times only induced half the levels of MT mRNA that were achieved by butyrate plus metals. Butyrate enhanced the rate of accumulation of MT mRNA in response to metals, increased the sensitivity of the MT gene to metals, and protected cells from toxic effects of high concentrations of metals. The butyrate and metal ion responses were selective in that no accumulation of c-myc, c-fms, HSP-70, or AFP mRNA was detected. However, c-fos mRNA accumulated in cells exposed to toxic concentrations of metals (50 microM and higher) and this was also potentiated by butyrate treatment. These results suggest that butyrate alters the chromatin conformation of both the MT-I and MT-II genes leading to an accentuated transcriptional response to metals.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Evans M. J., Magrane G. G. Biochemical markers of the progress of differentiation in cloned teratocarcinoma cell lines. Eur J Biochem. 1977 Oct 3;79(2):607–615. doi: 10.1111/j.1432-1033.1977.tb11845.x. [DOI] [PubMed] [Google Scholar]
- Adamson E. D., Gaunt S. J., Graham C. F. The differentiation of teratocarcinoma stem cells is marked by the types of collagen which are synthesized. Cell. 1979 Jul;17(3):469–476. doi: 10.1016/0092-8674(79)90254-x. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Adamson E. D., Gedamu L. The ontogeny of expression of murine metallothionein: comparison with the alpha-fetoprotein gene. Dev Biol. 1984 Jun;103(2):294–303. doi: 10.1016/0012-1606(84)90317-8. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Teng C. S. Studies on sex-organ development. Prenatal effect of oestrogenic hormone on tubular-gland cell morphogenesis and ovalbumin-gene expression in the chick Müllerian duct. Biochem J. 1979 Aug 15;182(2):271–286. doi: 10.1042/bj1820271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Pöting A., Mallick U., Rahmsdorf H. J., Schorpp M., Herrlich P. Induction of metallothionein and other mRNA species by carcinogens and tumor promoters in primary human skin fibroblasts. Mol Cell Biol. 1986 May;6(5):1760–1766. doi: 10.1128/mcb.6.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakka A., Webb M. Metabolism of zinc and copper in the neonate: changes in the concentrations and contents of thionein-bound Zn and Cu with age in the livers of the newborn of various mammalian species. Biochem Pharmacol. 1981 Apr 1;30(7):721–725. doi: 10.1016/0006-2952(81)90157-x. [DOI] [PubMed] [Google Scholar]
- Birren B. W., Herschman H. R. Regulation of the rat metallothionein-I gene by sodium butyrate. Nucleic Acids Res. 1986 Jan 24;14(2):853–867. doi: 10.1093/nar/14.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Warren R., Sarthy A., Palmiter R. D. Regulation of metallothionein--thymidine kinase fusion plasmids injected into mouse eggs. Nature. 1982 Mar 4;296(5852):39–42. doi: 10.1038/296039a0. [DOI] [PubMed] [Google Scholar]
- Cain K., Griffiths B. L. A comparison of isometallothionein synthesis in rat liver after partial hepatectomy and parenteral zinc injection. Biochem J. 1984 Jan 1;217(1):85–92. doi: 10.1042/bj2170085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Donner L., Fedele L. A., Garon C. F., Anderson S. J., Sherr C. J. McDonough feline sarcoma virus: characterization of the molecularly cloned provirus and its feline oncogene (v-fms). J Virol. 1982 Feb;41(2):489–500. doi: 10.1128/jvi.41.2.489-500.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Induction of metallothionein-I mRNA in cultured cells by heavy metals and iodoacetate: evidence for gratuitous inducers. Mol Cell Biol. 1984 Mar;4(3):484–491. doi: 10.1128/mcb.4.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. A., Adamson E. D. Induction of c-fos and AFP expression in a differentiating teratocarcinoma cell line. Exp Cell Res. 1986 Aug;165(2):473–480. doi: 10.1016/0014-4827(86)90600-2. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Stark G. R. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985 Apr 18;314(6012):637–639. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Ghosh M. K., Cox R. P. Production of human chorionic gonadotropin in HeLa cell cultures. Nature. 1976 Feb 5;259(5542):416–417. doi: 10.1038/259416a0. [DOI] [PubMed] [Google Scholar]
- Grover A., Adamson E. D. Roles of extracellular matrix components in differentiating teratocarcinoma cells. J Biol Chem. 1985 Oct 5;260(22):12252–12258. [PubMed] [Google Scholar]
- Grover A., Oshima R. G., Adamson E. D. Epithelial layer formation in differentiating aggregates of F9 embryonal carcinoma cells. J Cell Biol. 1983 Jun;96(6):1690–1696. doi: 10.1083/jcb.96.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager L. J., Palmiter R. D. Transcriptional regulation of mouse liver metallothionein-I gene by glucocorticoids. Nature. 1981 May 28;291(5813):340–342. doi: 10.1038/291340a0. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Walling M. Regulation in vivo of a cloned mammalian gene: cadmium induces the transcription of a mouse metallothionein gene in SV40 vectors. J Mol Appl Genet. 1982;1(4):273–288. [PubMed] [Google Scholar]
- Hammer R. E., Brinster R. L., Rosenfeld M. G., Evans R. M., Mayo K. E. Expression of human growth hormone-releasing factor in transgenic mice results in increased somatic growth. 1985 May 30-Jun 5Nature. 315(6018):413–416. doi: 10.1038/315413a0. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Karin M. Metallothioneins: proteins in search of function. Cell. 1985 May;41(1):9–10. doi: 10.1016/0092-8674(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Sone T., Otaki N., Kimura M. Cd2+-induced synthesis of metallothionein in HeLa cells. Biochem J. 1985 May 1;227(3):879–886. doi: 10.1042/bj2270879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Levine R. A., Campisi J., Wang S. Y., Gudas L. J. Butyrate inhibits the retinoic acid-induced differentiation of F9 teratocarcinoma stem cells. Dev Biol. 1984 Oct;105(2):443–450. doi: 10.1016/0012-1606(84)90301-4. [DOI] [PubMed] [Google Scholar]
- Low M. J., Hammer R. E., Goodman R. H., Habener J. F., Palmiter R. D., Brinster R. L. Tissue-specific posttranslational processing of pre-prosomatostatin encoded by a metallothionein-somatostatin fusion gene in transgenic mice. Cell. 1985 May;41(1):211–219. doi: 10.1016/0092-8674(85)90075-3. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Palmiter R. D. Glucocorticoid regulation of metallothionein-I mRNA synthesis in cultured mouse cells. J Biol Chem. 1981 Mar 25;256(6):2621–2624. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune Y., Kume A., Ogiso Y., Matsushiro A. Induction of teratocarcinoma cell differentiation. Effect of the inhibitors of DNA synthesis. Exp Cell Res. 1983 Jul;146(2):439–444. doi: 10.1016/0014-4827(83)90147-7. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Ovalbumin messenger ribonucleic acid translation. Comparable rates of polypeptide initiation and elongation on ovalbumin and globin messenger ribonucleic acid in a rabbit reticulocyte lysate. J Biol Chem. 1973 Mar 25;248(6):2095–2106. [PubMed] [Google Scholar]
- Plesko M. M., Hargrove J. L., Granner D. K., Chalkley R. Inhibition by sodium butyrate of enzyme induction by glucocorticoids and dibutyryl cyclic AMP. A role for the rapid form of histone acetylation. J Biol Chem. 1983 Nov 25;258(22):13738–13744. [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Senear A. W., Palmiter R. D. Expression of the mouse metallothionein-I gene alters the nuclease hypersensitivity of its 5' regulatory region. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):539–547. doi: 10.1101/sqb.1983.047.01.063. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Chen H. Y., Brinster R. L., Palmiter R. D. A 12-base-pair DNA motif that is repeated several times in metallothionein gene promoters confers metal regulation to a heterologous gene. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7318–7322. doi: 10.1073/pnas.81.23.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W., Simmons D. M., Arriza J., Hammer R., Brinster R., Rosenfeld M. G., Evans R. M. Novel developmental specificity in the nervous system of transgenic animals expressing growth hormone fusion genes. 1985 Sep 26-Oct 2Nature. 317(6035):363–366. doi: 10.1038/317363a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkle W. B., Tate C. A., Bick R. J., Entman M. L. Nucleotide triphosphate utilization by cardiac and skeletal muscle sarcoplasmic reticulum. Evidence for a hydrolysis cycle not coupled to intermediate acyl phosphate formation and calcium translocation. J Biol Chem. 1981 Mar 10;256(5):2268–2274. [PubMed] [Google Scholar]
- Yagle M. K., Palmiter R. D. Coordinate regulation of mouse metallothionein I and II genes by heavy metals and glucocorticoids. Mol Cell Biol. 1985 Feb;5(2):291–294. doi: 10.1128/mcb.5.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]