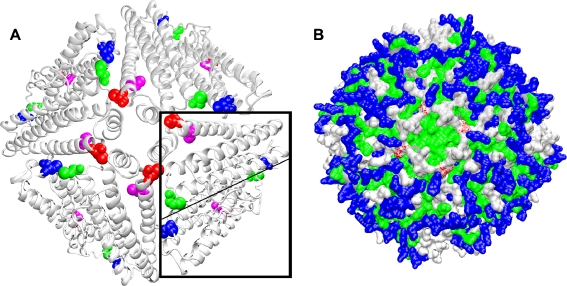
Fig. 7.
Cysteine localization within an artemin octomer model. An octomer of artemin monomers was modeled with MODELER 7 × 7 using the crystal structure of bullfrog ferritin. Residues 21-192 and 2-189, respectively, of artemin and ferritin were used for modeling. a Artemin octomer showing the positions of C22 (blue), C61 (green), C166 (magenta), and C172 (red). An artemin dimer is boxed and the monomer–monomer interface is indicated by the light line. b C172 (red) is shown within a hydrophobic region formed by four helices of neighboring subunits as shown in a. Polar charged residues (blue), polar non-charged residues (silver), hydrophobic residues (green). Images generated with VMD and Raster3D are in new cartoon (a) and surf (b)