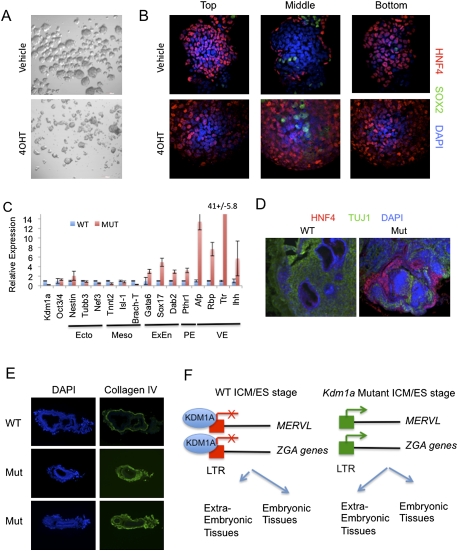
Figure 7.
Kdm1a mutant ES cells have increased potency to generate extraembryonic lineages. (A,B) Kdm1a FL/FL Cre-ERT ES cells were treated with 4OHT and maintained for 48 h before growing in bacterial-grade dishes in the absence of LIF to induce differentiation. (A) After 48 h, embryoid bodies were imaged by phase-contrast microscopy. After 8 d of differentiation, embryoid bodies were immunostained with HNF4 and SOX2 antibodies and overlaid with DAPI. Optical confocal sections were then taken through the entire embryoid body. (C) qRT–PCR analysis with the indicated primers was performed on tumors derived from Kmd1a Fl/FL CreERT ES cells treated with vehicle (WT) or 4OHT (Mut). Error bars represent SD. (Ecto) Ectoderm; (Meso) mesoderm; (ExEn) extraembryonic endoderm; (PE) parietal endoderm; (VE) visceral endoderm. (D) Tumors derived from Kdm1a wild-type (WT) and mutant (Mut) ES cells were immunostained with the VE marker HNF4 and the neural marker TUJ1 and counterstained with DAPI. (E) Cryosections from Kdm1a wild-type (WT) and KO/KO (Mut) embryos at embryonic day 6.5 were immunostained with anti-collagen IV antibodies and counterstained with DAPI. (F) Model of KDM1A function in the coordinated repression of MERVL and extraembryonic cell fate potential. In ES cells derived from the ICM, MERVL retroviruses and a subset of ZGA genes that are LTR-linked are repressed by KDM1A, and cell fate is restricted to embryonic lineages. In Kdm1a mutants, MERVL and LTR-linked ZGA genes are improperly activated, resulting in an expanded fate potential to generate extraembryonic tissues.