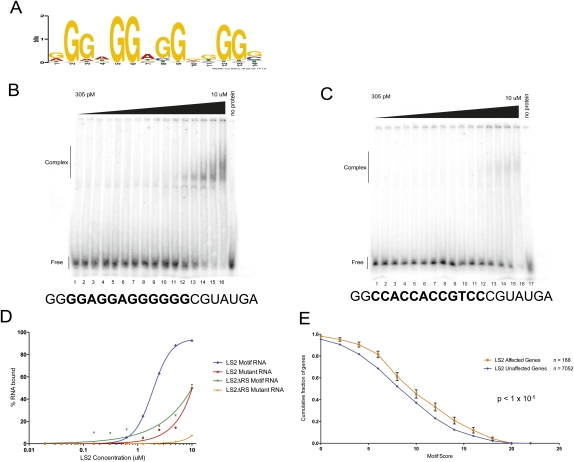
Figure 2.
LS2 has diverged in RNA-binding sequence specificity from dU2AF50, and its binding motif is enriched in its target transcripts. (A) SELEX-derived PSSM (position-specific scoring matrix) of the RNA sequence recognized by the LS2 protein. (B,C) Electrophoretic gel mobility shift assays using purified recombinant GST-tagged LS2 protein and a synthetic RNA containing the SELEX-derived G-rich recognition motif (B) or a mutant RNA in which all of the important guanosine residues (see motif) had been mutated to cytosine (C). Protein concentrations ranged from 305 pM (lane 1) to 10 μM (lane 16) in twofold increments. (Lane 17) No protein control. (D) PhosphorImager quantification of the results in A and B. Similar experiments were done using GST-tagged truncated versions of LS2 lacking the N-terminal RS domain (data not shown). (E) Enrichment of the LS2 recognition motif in its affected target transcripts. Each point represents the fraction of genes that contain an LS2 recognition motif scoring at the X-axis value or higher.