Figure 3.
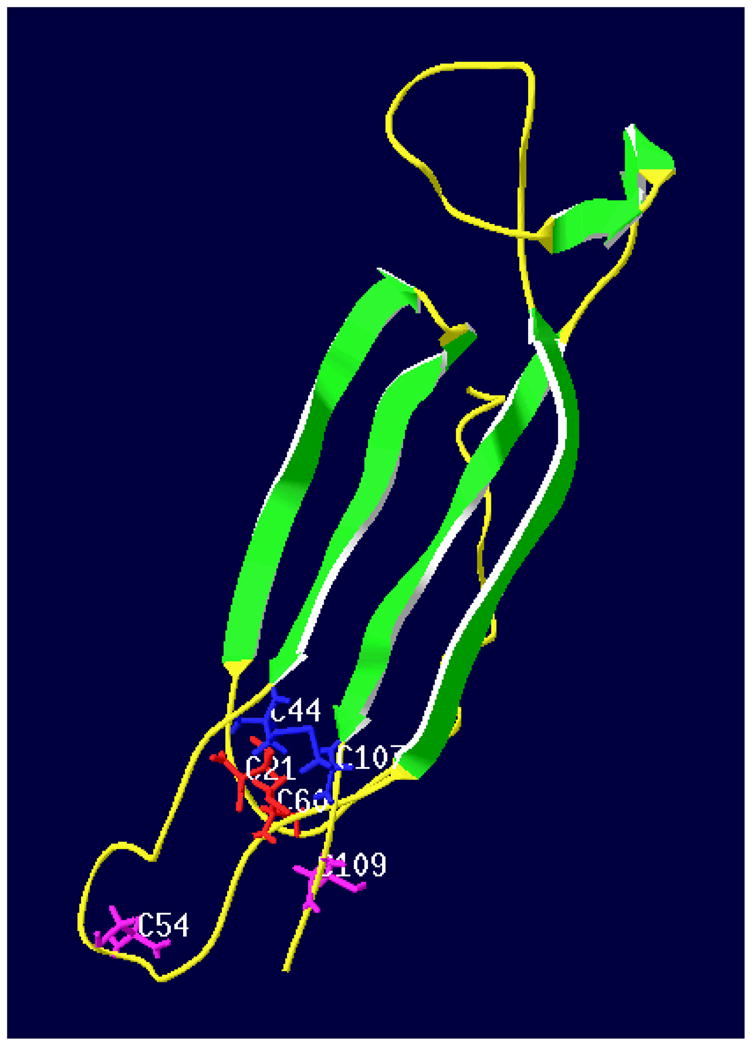
Three-dimensional structure of Cupip-PTTH. Green ribbons represent 6 β-strands including two β-sheets, each containing two antiparallel β-strands. The β-strands are arrowed from N-terminal to C-terminal. Yellow threads indicate coil areas. Six cysteines form three intra-chain disulfate bonds shown as C21, C44, C54, C60, C107, and C109. Groups including side chains of cysteines are colored in red (C21 and C60), blue (C44 and C107), and purple (C54 and C109) to show the formation of the three disulfate bonds. Predicted by I-TASSER program and viewed by SWISS-Pdb Viewer Deep View v4.0.1.
