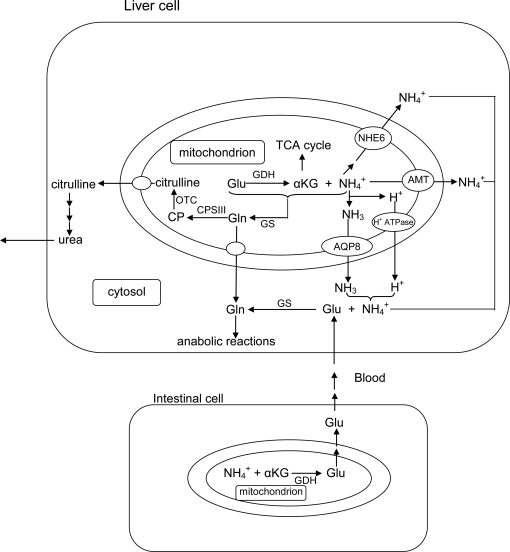
Figure 1.
The catabolism of excess amino acids through the process of transdeamination in liver cells releases ammonia and α-ketoglutarate (αKG) from glutamate (Glu) catalyzed by glutamate dehydrogenase (GDH) in the mitochondria. To avoid the disruption of the H+ gradient set up across the inner mitochondrial membrane by the electron transport system, ammonia produced within the mitochondrial matrix can exit the mitochondrion as through a putative transporter (AMT) or a Na+/H+ exchanger (e.g., NHE6) in the liver cells of ammonotelic fishes. Alternately, ammonia can exit through an aquaporin channel (e.g., AQP8) as NH3, accompanied with proton (H+) transport through an H+-ATPase. Ammonia can also be detoxified through the mitochondrial glutamine synthetase (GS) to glutamine (Gln), which is proton-neutral, before permeating the inner mitochondrial membrane to support various anabolic reactions (e.g., purine and pyrimidine syntheses). In ureogenic fishes that possess a functional ornithine-urea cycle, Gln synthesized within the mitochondria can be converted to carbamoyl phosphate (CP) catalyzed by carbamoyl phosphate synthetase III (CPS III) and subsequently to citrulline catalyzed by ornithine transcarbamylase (OTC) in the mitochondrial matrix of liver cells. Citrulline, being proton-neutral, can permeate the inner mitochondrial membrane without disrupting the H+ gradient. Some fishes (e.g., Bostrychus sinensis) possess a high activity of cytosolic GS in the liver (and the muscle), which would facilitate the detoxification of both endogenous and exogenous ammonia to glutamine in the cytosol, with glutamate being supplied from intestinal cells to support increased glutamine synthesis.