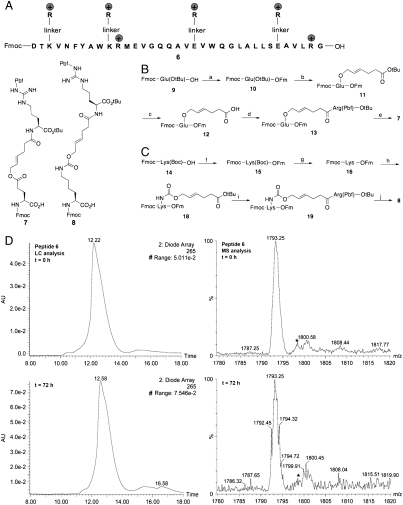
Fig. 4.
Preparation and characterization of peptide 6. (A) The sequence of peptide 6. (B) The synthesis of building block 7. Pbf = 2,2,4,6,7-Pentamethyldihydrobenzofuran-5-sulfonyl. Key: (a) Fm-OH, DCC, DMAP, CH2Cl2, 20 min, 99%; (b) TFA∶CH2Cl2 (1∶1), 30 min; BrCH2CH = CHCH2CH2CO2tBu, Bu4NBr, NaHCO3, H2O, CH2Cl2, THF, 7.5 h, 60%; (c) TFA∶CH2Cl2 (1∶1), 35 min, 86%; (d) H-Arg(Pbf)-OtBu, DCC, DMAP, CH2Cl2, 30 min, 74%; (e) Piperidine, CH2Cl2, 20 min; Fmoc-OSu, NaHCO3, H2O, THF, 2.5 h, 29%. (C) The synthesis of building block 8. Key: (f) Fm-OH, DCC, DMAP, CH2Cl2, 20 min, 28%; (g) 4M HCl in dioxane, 1 h, 50%; (h) SuOC( = O)OCH2CH = CHCH2CH2CO2tBu 17, NaHCO3, H2O, CH3CN, 1 h, 40%; (i) TFA∶CH2Cl2 (1∶1), 40 min; H-Arg(Pbf)-OtBu, DCC, DMAP, CH2Cl2, 10 min, 50%; (j) Piperidine, CH2Cl2, 10 min; Fmoc-OSu, NaHCO3, H2O, THF, 2 h, 94%. (D) LC-MS analysis of synthetic peptide 6.