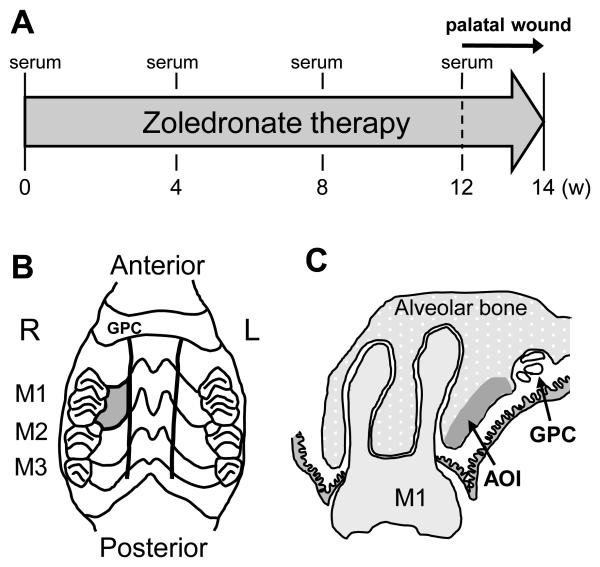
Fig. 1.
Study design, palatal wounds and bone area of interest. A: Serum was collected at 0, 4, 8, and 12 weeks after the initiation of zoledronate therapy. The palatal bone was exposed to the oral cavity at 12 weeks after the initiation of zoledronate administration. Zoledronate therapy continued until the end of study (week-14). B: The palatal mucosa confined by the rugae, the first molar (M1), and the great palatine canal (GPC) was excised and the alveolar bone denuded (gray-colored area). C: The alveolar bone was assessed for osteocyte lacunae at the study end. Area of interest (AOI) was defined as the bone area within 1.0mm from the alveolar bone surface and between the M1 and GPC.