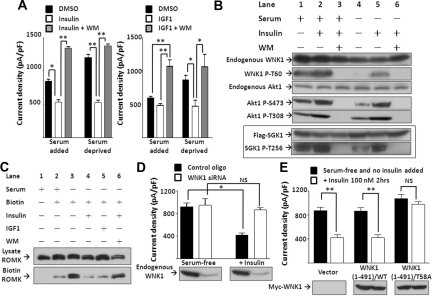
Figure 2.
Insulin and IGF1 inhibit ROMK through PI3K and WNK1–T58 phosphorylation. (A) Effect of insulin and IGF1 on ROMK is blocked by wortmannin. Cells cultured with or without serum were treated by DMSO, insulin 100 nM, or insulin 100 nM plus wortmannin (WM, 100 nM), respectively, for 2 hours (mean ± SEM, n ≥ 6). (B) Effect of serum, insulin, and wortmannin on phosphorylation of endogenous WNK1, Akt1, and overexpressed SGK1. Phosphorylation on specific residues was determined by specific anti-phospho antibodies. Shown is representative of three separate experiments of similar results. (C) Effect of insulin and IGF1 on surface abundance of ROMK. Cells were serum-deprived for 16 hours and treated with insulin (100 nM), IGF1 (100 ng/ml), and/or WM (100 nM) for 2 hours as indicated before biotinylation (Biotin). ROMK in total cell lysates (Lysate ROMK) and elute from avidin beads (Biotin ROMK) were detected by Western blot. (D) Insulin inhibits ROMK through WNK1. Cells transfected with control oligonucleotide or WNK1 siRNA (200 nM each) were deprived of serum for 16 hours (serum-free group). Insulin (100 nM) was added for 2 hours (+Insulin group). Successful knockdown of endogenous WNK1 by siRNA is evident by Western blot analysis. Mean ± SEM (n ≥ 6 each). (E) Insulin inhibits ROMK through phosphorylation on T58 of WNK1. Cells transfected with empty vector, wild-type (WT), or T58A mutant WNK1(1-491) were cultured in serum-free medium for 16 hours and received insulin (100 nM) or not for 2 hours. Equal amount of WT or T58A WNK1(1-491) expression is evident by Western blot analysis. Mean ± SEM (n ≥ 6 each). In A, D, and E, *P < 0.05 between indicated groups by unpaired two-tailed t test. **P < 0.01. NS, not statistically significant.