Abstract
Historically, patients with type 1 diabetes and macroalbuminuria had high competing risks: cardiovascular death or renal failure. Here, we assessed these risks in patients receiving therapies implemented during the last 30 years. Between 1991 and 2004, we enrolled 423 white patients with type 1 diabetes who developed macroalbuminuria (albumin excretion rate, ≥300 μg/min). With follow-up for 98% through 2008, ESRD developed in 172 patients (incidence rate, 5.8/100 person-years), and 29 died without ESRD (mortality rate, 1/100 person-years). The majority of these outcomes occurred between ages 36 and 52 years with durations of diabetes of 21 to 37 years. The 15-year cumulative risks were 52% for ESRD and 11% for pre-ESRD death. During the 15 years of follow-up, the use of renoprotective treatment increased from 56 to 82%, and BP and lipid levels improved significantly; however, the risks for both ESRD and pre-ESRD death did not change over the years analyzed. There were 70 post-ESRD deaths, and the mortality rate was very similar during the 1990s and the 2000s (11/100 person-years versus 12/100 person-years, respectively). Mortality was low in patients who received a pre-emptive kidney transplant (1/100 person-years), although these patients did not differ from dialyzed patients with regard to predialysis eGFR, sex, age at onset of ESRD, or duration of diabetes. In conclusion, despite the widespread adoption of renoprotective treatment, patients with type 1 diabetes and macroalbuminuria remain at high risk for ESRD, suggesting that more effective therapies are desperately needed.
Persistent macroalbuminuria develops in one in three patients with type 1 diabetes (T1D) during their lifetime.1,2 Its appearance has been viewed as the beginning of progressive renal function loss and impending failure or ESRD. Before universal availability of renal replacement therapy in the mid-1970s, patients who developed ESRD died shortly thereafter, typically because of uremia or cardiovascular death.1–3
During the last 30 years, care of T1D patients with macroalbuminuria underwent major changes. Aggressive treatment of hypertension and renoprotective blockade of the renin-angiotensin system were implemented to mitigate deterioration of renal function.4–7 Access to renal replacement therapy was gained through Medicare, and significant advances were made in the metabolic management of patients on dialysis. Patients with renal transplant have new immunosuppressant medications and protocols that reduced graft rejection and increased patient survival.8,9 Also a strategy of “pre-emptive” renal transplantation has been adopted to avoid dialysis and its associated high mortality.10,11 Similarly, efforts to reduce cardiovascular mortality through treatment of hypercholesterolemia and hypertension and by encouraging smoking cessation may have reduced mortality both pre- and post-ESRD. Little research has been done to determine the magnitude of the effect of all of these protocols on the course of the advanced stages of diabetic nephropathy in T1D.
The primary goal of this study was to determine the modern-day clinical course of T1D with macroalbuminuria; that is, to assess the risk of ESRD and magnitude of pre- and post-ESRD mortality in 423 patients recruited into the study between 1991 and 2004 and followed through 2008. The majority of these patients had been attending the Joslin Clinic since soon after diagnosis of diabetes, and their care included prompt incorporation of the evolving recommendations and new protocols for treating patients with T1D and diabetic nephropathy, including its advanced stage macroalbuminuria. A second goal of the study was to identify predictors of the development of ESRD to enable better risk assessment of patients and optimal selection of clinical protocols.
RESULTS
Secular Trends in Characteristics of Patients with Macroalbuminuria
The Joslin Clinic provides long-term care for about 3500 adult patients with T1D. We screened a random half of this population in 1991 to 1992 for albuminuria as part of the Joslin Study on the Natural History of Microalbuminuria.12 Albuminuria was categorized into normo-, micro-, or macroalbuminuria on the basis of multiple measurements of the urinary albumin to creatinine ratio (ACR) in random urines (see Concise Methods for ACR criteria for defining macroalbuminuria). Patients diagnosed with macroalbuminuria by this screening were recruited into our study of the genetics of kidney disease in T1D between 1991 and 2000. During subsequent follow-up of this cohort, additional patients with macroalbuminuria were detected by systematic rescreening, and they were recruited as well. Between 2001 and 2004, the remaining patients with T1D and macroalbuminuria attending the Joslin Clinic were recruited for this study. The analysis in this report was restricted to the 423 patients out of the 457 enrolled who identified themselves as white.
To facilitate description of trends, the 15-year span of enrollment was divided into three 5-year subcohorts: 1991 to 1995, 1996 to 2000, and 2001 to 2004. Characteristics of patients at entry are summarized in Table 1 according to subcohort. A trend in median age from 34 to 42 years (P < 0.0001) was paralleled by longer T1D duration (23 to 27 years, P = 0.61 adjusted for age). All of the patients had remained under Joslin Clinic care for almost 20 years but glycemic control (HbA1c) was poor for all. Independent of the age trend, several characteristics changed with calendar time, as did use of certain treatments (Table 1): BMI increased (P < 0.001); use of lipid-lowering drugs increased (P < 0.0016), and total cholesterol improved (P = 0.0001); use of angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin-receptor blockers (ARB) increased from 56 to 82% (P < 0.0001), and BP decreased (systolic 137 to 131 mmHg, P < 0.0016; diastolic 81 to 76 mmHg, P = 0.0001). ACR did not change. Consistent with the older age of the last subcohort, their average eGFR at enrollment was significantly lower (P = 0.005 but P = 0.67 after adjustment for age).
Table 1.
Characteristics of patients with T1D and macroalbuminuria according to subcohort defined by date of enrollment
| Characteristic | Subcohort |
P | ||
|---|---|---|---|---|
| 1991 to 1995 (n = 113) | 1996 to 2000 (n = 112) | 2001 to 2004 (n = 198) | ||
| Men (%) | 53.1 | 53.6 | 53.5 | 1.0 |
| Age (years) | 34.1 ± 5.7 | 37.6 ± 7.2 | 41.8 ± 9.7 | <0.0001 |
| Age at T1D diagnosis (years) | 11.5 ± 6.7 | 11.6 ± 7.2 | 15.3 ± 8.9 | <0.0001 |
| T1D duration (years) | 22.6 ± 6.7 | 26.0 ± 8.2 | 26.5 ± 8.9 | 0.0001 |
| Duration of care at Joslin (years) | 17.4 ± 8.8 | 21.2 ± 10.7 | 18.0 ± 12.0 | 0.05 |
| HbA1c (%) | 9.1 ± 1.6 | 9.4 ± 1.9 | 8.7 ± 1.6 | 0.01 |
| Body mass index (kg/m2) | 25.1 ± 4.3 | 25.3 ± 4.8 | 27.6 ± 6.4 | <0.0001 |
| Patients using lipid-lowering drugs (%) | 8.0 | 10.7 | 42.4 | <0.0001 |
| Total cholesterol (mg/dl) | 228 ± 64 | 212 ± 51 | 202 ± 49 | <0.0001 |
| Current smokers (%) | 28.1 | 26.4 | 20.7 | 0.30 |
| Systolic blood pressure (mmHg) | 137 ± 20 | 134 ± 18 | 131 ± 17 | 0.006 |
| Diastolic blood pressure (mmHg) | 81 ± 10 | 80 ± 9 | 76 ± 10 | <0.0001 |
| Patients using ACE-I or ARB (%) | 55.8 | 62.5 | 81.8 | <0.0001 |
| ACR (mg/g) | 967 (526 to 1667) | 736 (442 to 1310) | 778 (465 to 1795) | 0.15 |
| eGFR (ml/min) | 70 ± 35 | 73 ± 37 | 60 ± 30 | 0.005 |
The data are the means ± SD or percent. eGFR, MDRD estimate of glomerular filtration rate based on serum creatinine.
Risk of ESRD
To examine whether the secular improvements in treatment of hypertension, lipid abnormalities, and achievement of almost universal treatment with renoprotective drugs had any effect on risk of ESRD, the analysis was restricted to the first 6 years after entry, an interval equivalent for all three subcohorts (Table 2). The incidence rate of ESRD did not vary significantly in any subcohort according to treatment with ACE-I/ARB nor among subcohorts in either treatment category. Difference among subcohorts in the risk of ESRD during the first 6 years of follow-up were not statistically significant in Cox models that adjusted for eGFR, HbA1c, ACR, gender, age, duration of diabetes, systolic BP, body mass index, total cholesterol, HDL cholesterol, and smoking status. Hazard ratios (95% confidence interval [CI]) for the 1996 to 2000 and 2001 to 2004 subcohorts relative to the 1991 to 1995 subcohort were 0.91 (0.54 to 1.50) and 0.88 (0.57 to 1.37), respectively.
Table 2.
Risk of ESRD and pre- or post-ESRD mortality in patients with T1D and macroalbuminuria according to subcohort defined by date of enrollment
| Subcohort |
|||
|---|---|---|---|
| 1991 to 1995 (n = 113) | 1996 to 2000 (n = 112) | 2001 to 2004 (n = 198) | |
| First 6 years of follow-up | |||
| number of person-years | 517 | 561 | 864 |
| incidence rate of ESRD per 100 person-years | |||
| in patients using ACE-I/ARB | 6.2 (20) | 6.1 (21) | 6.4 (46) |
| in patients not using ACE-I/ARB | 6.5 (16) | 4.9 (11) | 6.3 (9) |
| in all patients | 6.3 (36) | 5.7 (32) | 6.4 (55) |
| age at ESRD onset (years) | 39 (36, 46) | 42 (38, 46) | 46 (38, 52) |
| T1D duration at ESRD onset (years) | 24 (21, 30) | 30 (22, 37) | 28 (23, 37) |
| All years of follow-upa | |||
| number of person-years | 1175 | 888 | 902 |
| incidence rate of ESRD per 100 person-years | 5.3 (62) | 5.5 (49) | 6.8 (61) |
| pre-ESRD mortality rate per 100 person-years | 0.8 (9) | 1.1 (10) | 1.1 (10) |
| number of person-years | 332 | 180 | 135 |
| post-ESRD mortality rate per 100 person-years | 10.3 (34) | 10.6 (19) | 12.6 (17) |
The data are the incidence rates (number of events) or medians (25th, 75th percentiles).
aFollow-up of the cohort of 423 patients through 2008 gave observation intervals with a median (25th, 75th percentiles) of 6.9 (5.2, 12.0) years and 3612 person-years of observation (2966 person years with macroalbuminuria and 646 person-years after ESRD onset).
Paralleling the increasing age at entry, the age at ESRD onset also increased in successive subcohorts (P = 0.002 and P = 0.06 after adjustment for entry age). This was accompanied by an increase in the duration of T1D at ESRD onset. Too few pre-ESRD deaths occurred to evaluate these characteristics for pre-ESRD mortality.
The absence of a difference between subcohorts in the incidence rate or cumulative incidence persisted throughout follow-up, so further analyses combined the experience of all three cohorts (Table 2). During 2966 person years of follow-up, ESRD developed in 172 patients for an incidence rate of 5.8 patients per 100 person-years (95% CI: 5.0, 6.7). The cumulative risk of ESRD at 5, 10, and 15 years was 24.4% (SE = 2.1%), 43.0% (SE = 2.9%), and 52% (SE = 3.5%), respectively.
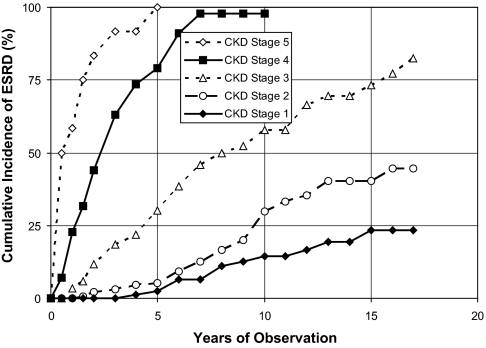
Risk of ESRD was strongly associated with chronic kidney disease (CKD) stage at enrollment. Within 5 years, ESRD developed in 100% of patients with stage 5 CKD and 79% of those with stage 4 CKD (Figure 1). By contrast, ESRD developed in few patients in stage 1 (2.3%) or stage 2 (5.3%) in that time. The risk for those in stage 3 was intermediate: 30% within 5 years. After 10 years of follow-up, the cumulative incidences of ESRD for CKD stages 4, 3, 2, and 1 were 98, 58, 30, and 14%, respectively.
Figure 1.
Cumulative incidence of ESRD in patients with T1D and macroalbuminuria rises with increased baseline CKD stages.
Other Predictors of ESRD
Except for patients in stages 4 and 5, ample opportunity remained before the onset of ESRD in the others to attempt postponement of renal failure by modifying determinants of progression. Therefore, we examined the determinants of time to onset of ESRD in stages 1 through 3 with Cox proportional hazard models.
Only three of the covariates in Table 1 were significantly associated with time to onset to ESRD in multivariate Cox proportional hazard models (Table 3). The risk of ESRD increased with CKD stage through stages 1 through 3 (hazard ratio [HR] = 2.85 for one stage increase) and with increase of baseline HbA1c and ACR. The effects of a 1% increase in HbA1c and a doubling of ACR were approximately equivalent. We did not observe significant effect modification of any variable by the others.
Table 3.
HRs for baseline characteristics of patients with T1D and macroalbuminuria (CKD stages 1 to 3 only) in Cox proportional hazard analysis of time to onset of ESRD
| Baseline Characteristic | Univariate Model |
Multivariate Model |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| One-stage increase of CKD | 2.85 | 2.13 to 3.80 | <0.0001 | 2.85 | 2.13 to 3.82 | <0.0001a |
| 1% increase of HbA1c | 1.24 | 1.11 to 1.39 | <0.0002 | 1.32 | 1.17 to 1.48 | <0.0001a |
| Doubling of ACR | 1.82 | 1.54 to 2.16 | <0.0001 | 1.68 | 1.42 to 1.99 | <0.0001a |
| Sex (reference female) | 0.73 | 0.50 to 1.07 | 0.11 | |||
| Age (10-year increase) | 1.05 | 0.82 to 1.34 | 0.70 | |||
| Diabetes duration (10-year increase) | 0.90 | 0.70 to 1.15 | 0.39 | |||
| Systolic blood pressure (10-mmHg increase) | 1.02 | 0.91 to 1.13 | 0.74 | |||
| BMI (1-unit increase) | 0.98 | 0.94 to 1.02 | 0.35 | |||
| Total cholesterol (20-mg/dl increase) | 1.12 | 1.03 to 1.21 | 0.006 | 0.99 | 0.91 to 1.08 | 0.89a |
| HDL cholesterol (10-mg/dl decrease) | 1.04 | 0.92 to 1.17 | 0.58 | |||
| Smoking (reference nonsmokers) | 1.34 | 0.87 to 2.06 | 0.18 | |||
| Statin use (reference not treated) | 1.82 | 1.12 to 2.96 | 0.02 | 1.49 | 0.91 to 2.45 | 0.12a |
The effect of CKD stage was uniform across the three stages tested. The quadratic and cubic terms for continuous variables were not significant. There were no significant interactions between regressors.
aVariable included in the multivariate model.
Pre- and Post-ESRD Mortality
During 2966 person-years of follow-up, 29 patients died without ESRD (pre-ESRD) for a mortality rate of 1.0 (95% CI 0.7, 1.4) per 100 person-years. The predicted number of deaths was 9.5, assuming the all-cause mortality rates in the U.S. population in 2007. Thus, the observed number of deaths unrelated to ESRD in the Joslin cohort was three times higher than expected. This mortality did not vary among the three subcohorts (Table 2). Cumulative pre-ESRD mortality at 5, 10, and 15 years was 2.7% (SE = 2.1%), 6.9.0% (SE = 2.9%), and 11.1% (SE = 3.5%), respectively. As shown in Table 4, patients in CKD stage 4 or 5 did not experience pre-ESRD death, whereas patients in stages 1 through 3 experienced similar risks of pre-ESRD death. A majority (69%) of pre-ESRD deaths were attributed to cardiovascular disease.
Table 4.
Mortality rate during macroalbuminuria according to CKD stage at enrollment in the study and mortality rate after onset of ESRD according the method of renal replacement therapy
| Person-years of Observation | Number of Deaths | Mortality Rate (per 100 Person-years) | |
|---|---|---|---|
| During macroalbuminuria (pre-ESRD) | |||
| stage 1 (n = 97) | 938 | 7 (4)b | 0.7 (0.4, 1.6)c |
| stage 2 (n = 135) | 1125 | 11 (6)b | 1.0 (0.5, 1.8)c |
| stage 3 (n = 122) | 734 | 11 (10)b | 1.5 (0.8, 2.7)c |
| stages 4 and 5 (n = 69) | 168 | 0 | 0 |
| After onset of ESRD | |||
| dialysis began before 2001 (n = 56) (30)a | 342 | 38 (27)b | 11.1 (8.1, 15.2)c |
| dialysis began after 2000 (n = 83) (31)a | 188 | 24 (17)b | 12.8 (8.6, 19.1)c |
| pre-emptive kidney transplant (n = 23) (23)a | 117 | 1 (0)b | 0.9 (0.1, 6.1)c |
| ESRD without dialysis (n = 10) (0)a | n.a. | 7 (4)b | n.a. |
n.a., not applicable.
aNumber of patients who received at least one kidney transplant.
bNumber of deaths caused by cardiovascular disease.
c95% CI.
Among the 172 patients who developed ESRD, dialysis began in 1991 to 2000 for 56 and in 2001 to 2008 for 83, and pre-emptive kidney transplants (without dialysis) were given to 23. The remaining ten developed renal failure but did not receive renal replacement therapy for various reasons. Within a year before the initiation of renal replacement therapy, serum creatinine was measured by the Joslin Clinical Laboratory for almost 60% of these patients. The eGFR estimated from these values was examined according to these three groups. The median (25th, 75th percentiles) for those starting dialysis in 1991 to 1999 or in 2000 to 2008 or having a pre-emptive kidney transplant were: 8.8 ml/min (7.6, 13.3), 11.0 ml/min (8.1, 16.0), or 9.5 ml/min (8.3, 14.5), respectively.
During 646 person-years of observation of 172 patients after the onset of ESRD (Table 4), 70 died for a mortality rate of 10.8 (95% CI 8.6, 13.7) per 100 person-years. The 10-year cumulative mortality after the onset of ESRD was 56.6% (SE = 5.3%). Again, a majority (69%) were attributed to cardiovascular disease. Post-ESRD mortality is summarized in Table 4 according to the same three subgroups. Mortality was similar regardless of whether ESRD developed before or after 2000. However, it was much lower for the 23 patients in the latter period who opted for a pre-emptive renal transplant rather than dialysis. Only one died during 117 person-years of subsequent follow-up, whereas 14 deaths were expected on the basis of the mortality in the other two groups (P = 0.0094). These 23 patients did not differ from dialyzed patients with regard to predialysis eGFR, gender, age at onset of ESRD, or duration of diabetes.
For comparisons with studies that did not distinguish pre- and post-ESRD mortality, we computed cumulative total mortality (all-cause and cardiovascular mortalities) disregarding whether ESRD intervened. At 5, 10, and 15 years, cumulative all-cause mortality was 10, 25, and 38%, and cumulative cardiovascular mortality was 8, 17, and 26%, respectively.
DISCUSSION
Our follow-up of a large cohort of patients with T1D and macroalbuminuria spotlights several unexpected findings. Foremost is the continued high risk of ESRD, which has not been diminished over the last 20 years by increased use of renoprotective drugs. Because the cohort received attentive health care at the Joslin Clinic, this cannot be attributed to poor access to medical expertise. On the other hand, mortality, which competes with ESRD to be the fate for these patients, emerges as a relatively minor competitor. This is true for both all-cause and cardiovascular mortality. However, after the onset of ESRD, mortality is very high despite the availability of renal replacement therapy. However, patients who receive pre-emptive kidney transplants (before dialysis) largely seem to be spared this excess mortality. Finally, the outcomes for patients with T1D and macroalbuminuria appear to vary among treatment centers. Although the current experience of patients of the Joslin Clinic is similar to that of patients in the FinnDiane study in Finland,13 it appears to contrast strikingly with that of a similar cohort of patients at Steno Memorial Hospital in Denmark.14
Before renoprotective therapies became standard care, macroalbuminuria heralded progression of nephropathy to ESRD, approximately 50% within 10 years and 75% within 15 years.1,2 Subsequent clinical studies and randomized trials examined the effectiveness of aggressive antihypertensive and renoprotective therapies and projected a less grim prognosis.6,7 Although clinical observations of the population treated at the Steno Memorial Hospital in Denmark amplified this message with evidence of diminishing risk of ESRD over time,14–16 the risk of ESRD in the Joslin cohort has remained undiminished despite almost universal renoprotective treatment. A similarly undiminished risk is reported for Finland.13 Furthermore, this undiminished risk seems to be true for nondiabetic CKD patients as well. For example in African Americans with hypertensive CKD, progression to ESRD appears relentless, even in a setting that achieved a low BP goal and almost universal renoprotective therapy.17
To facilitate consideration of similarities and differences that might account for disparate results among the three studies in T1D, Table 5 summarizes their characteristics and outcomes. The cohorts are similar with respect to calendar years of recruitment and main clinical characteristics. All had excellent follow-up. The risk of ESRD per 100 person-years was 5.8 in the Joslin cohort and 5.1 in the FinnDiane cohort but only 1.6 in the Steno study, which is only about 30% of that in the other cohorts. The risk of pre-ESRD mortality was low and identical in the first two cohorts but significantly higher in the Steno Study, although still much lower than that in type 2 diabetes.18
Table 5.
Clinical characteristics and outcomes of patients with type 1 diabetes and macroalbuminuria according to the origin of the cohort
| Joslin Cohort (n = 423) | FinnDiane Cohort (n = 592)13 | Steno Cohort (n = 397)14 | |
|---|---|---|---|
| Characteristic at enrollment | |||
| enrollment year | 1991 to 2004 | 1995 to 2006 | 1993 to 2005 |
| ascertainment | Clinic patients | Countrywide | Clinic patients and referralsa |
| age (years) | 38.7 ± 8.8 | 41.6 ± 10.2 | 42.1 ± 10.6 |
| duration of T1D (years) | 25.3 ± 8.3 | 29.4 ± 8.2 | 28.3 ± 8.9 |
| HbA1c (%) | 9.0 ± 1.7 | 9.0 ± 1.5 | 9.4 ± 1.5 |
| systolic BP (mmHg) | 133 ± 18 | 145 ± 20 | 145 ± 22 |
| albumin excretion rate (mg/24 h) | 797b | proteinuria in the past | 609b |
| eGFR (ml/min) | 66 ± 34 | 52 ± 26 | 67 ± 28 |
| using antihypertensive drug (%) | 70b | 95 | 75 |
| Outcome measures | |||
| duration of follow-up (years) | 6.9 (5.2, 12.0)c | 9.9 (7.3, 10.6)c | 11.3 (0 to 12.3)d |
| number of ESRD | 172 (5.8)e | 210 (5.1)e | 70 (1.6)e |
| number of pre-ESRD deaths | 29 (1.0)e | 56 (1.4)e | 98 (2.2)e,f |
aReferred for measurement of GFR.
bACE-I or ARB.
cMedian (25th, 75th percentile).
dMedian (range).
eNumber of events (rate per 100 person-years).
fEstimated number: total deaths were 126 without specification of how many were without ESRD. We assumed mortality after ESRD was the same as in the Joslin cohort, and this left 98 deaths pre-ESRD.
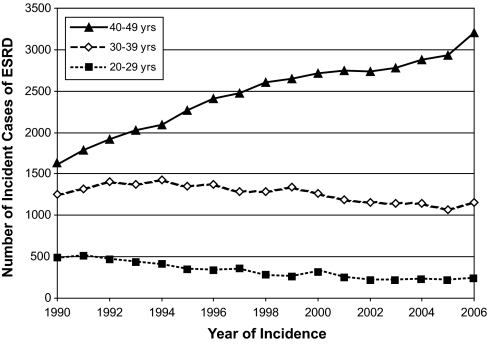
The persistent high risk of ESRD for patients with T1D and macroalbuminuria treated at Joslin is consistent with data for the U.S. population as a whole (Figure 2). The number of cases of ESRD caused by T1D in age categories 20 to 29 and 30 to 39 years has declined slightly but almost doubled for ages 40 to 49 years. The national trend toward an older age at onset is evident in the Joslin cohort (Table 2). The parallel increase in age at entry to the study suggests that most likely improved glycemic control19 has delayed the onset of macroalbuminuria to an older age. However, the absence of a temporal trend in risk of ESRD in the Joslin subcohorts indicates that this improvement has not affected progression to ESRD once macroalbuminuria has developed.
Figure 2.
Number of incident cases of ESRD attributable to diabetes according to calendar time increases in 40- to 49-year-olds and decreases in 20- to 39-year-olds. Total number of incident cases among 20- to 49-year-olds in 1990 was 3359; in 1995, it was 3972; in 2000, it was 4287; and in 2006, it was 4600. We obtained from the U.S. Renal Data System (http://www.usrds.org/) the number of incident cases of ESRD attributed to diabetes between 1990 and 2006 that occurred in the white U.S. population. In these patients, T1DM is the predominant diabetes type and almost the exclusive type in those with sufficient diabetes duration to develop diabetes associated ESRD.
Although the strong relationship of the risk of ESRD to CKD stage at enrollment was expected, our data are the first to provide contemporary estimates of the magnitude of these risks of ESRD in T1D patients with macroalbuminuria according to standardized estimates of GFR. These data are useful for advising patients about the probable time remaining before ESRD develops and for planning effective therapeutic strategies.
For patients in CKD stages 1 through 3, ESRD developed in only a minority within 5 years. Thus, ample time remains to attempt postponing the onset of ESRD for the majority of patients with T1D and macroalbuminuria. The two risk factors with the greatest influence on time to onset of ESRD were poor glycemic control and severity of macroalbuminuria. Whether interventions to reduce these exposures would modify the course of renal function loss is unknown. Equally uncertain is how patient care can be tailored to bring patients below, for example, their current medians (HbA1c, ≤9.0%; ACR, ≤762 mg/g).
It is apparent that the majority of patients with macroalbuminuria, despite remaining under the care of the Joslin Clinic for almost 20 years, had poor glycemic control at entry into the study. Therefore, new protocols to improve glycemic control (for example through an insulin pump program or pancreas transplant) should be tested for their effectiveness in retarding renal function loss and postponing the onset of ESRD. Such a clinical trial has just begun in Europe.20 In a small series of trials with patients with type T1D, long-term normal glycemic control induced by pancreas transplant reversed histologic lesions of diabetic nephropathy.21
Despite almost universal renoprotective treatment, there is a continuing high risk of ESRD in patients with T1D and macroalbuminuria. This points to the need for new, more effective therapies to lower the high levels of macroalbuminuria that were observed in the Joslin cohort or to target other causal pathways, which are involved in the progression to ESRD. At present, it is unclear whether the currently examined drugs such as renin inhibitors (e.g. aliskiren), protein kinase C inhibitors (ruboxistaurin mesylate), AGE inhibitors (e.g. pyridoxamine), or antifibrotic agents (e.g. pirfenidone) might have a strong effect to reduce the risk of ESRD in patients with macroalbuminuria in the Joslin cohort.22–26
All of the patients who entered the study in CKD 5 required renal replacement therapy within 5 years, as did the majority of those in stage 4. The poor prognosis for these patients justifies pursuing pharmacologic experiments that target signaling pathways and possibly intervening more aggressively to prolong renal function. This situation may be comparable with experimental therapies that target TGF-β signaling in the treatment of cancer.27,28
An alternative or complementary approach is to reduce the mortality once these patients develop ESRD. Only a few patients in our cohort were too ill for renal replacement therapy, but those able to start dialysis still had a poor prognosis, and it did not improve with calendar time. With a mortality rate of 11/100 person-years, a minority survived for 10 years. Interestingly, a group of patients in our study received pre-emptive kidney transplants and had excellent 5-year survival. Our findings seem to be better than those of other reports.10,11,29 Because our study group is small, our finding about the effectiveness of pre-emptive kidney transplantation in patients with T1D and ESRD needs to be seen as preliminary. The challenge is how to increase frequency of pre-emptive kidney transplantation in patients with T1D and ESRD.11 In the Joslin cohort such transplantation was implemented in 20% of patients who developed ESRD in the 2000s.
Limitations of our study need to be discussed. Because the diabetes care and treatment of macroalbuminuria at the Joslin Clinic may be better than that generally received by patients, the outcomes in our study may underestimate the risks elsewhere. Another limitation is that ascertainment of patients with macroalbuminuria in our study included both prevalent and incident cases. This may have diminished our ability to see a secular trend in the risk of ESRD in the Joslin cohort, despite Cox proportional analysis allowing us to control for possible confounders. Another limitation of our study is that the analysis was based on the baseline measurement of clinical covariates. Therefore, the effect of covariates that vary over time, such as BP, was not fully captured, and this limitation could account for a failure to detect an effect on the risk of ESRD. On the other hand the single measurement was sufficient to characterize patients for covariates that track well over time, such as ACR and HbA1c. Also the single determination of ACEI/ARB treatment might not be sufficient to detect a statistically significant effect of these drugs on risk of ESRD. However, incorporating changes over time in usage of these drugs, as we have done previously,30 most likely would not change our findings.
Finally, because renoprotective treatment became more prevalent in the source populations of successive subcohorts, later subcohorts might have been enriched with macroalbuminuric nonresponders to ACE-I/ARB, who might have had a higher risk of ESRD. However, if this were the case, we would have seen a declining incidence of ESRD caused by T1D in the U.S. population. Instead, the number of ESRD cases caused by T1D increased over the last 15 years (Figure 2). Moreover, in a previous publication we reported that patients with microalbuminuria who were treated with ACE inhibitors in the Joslin Clinic setting actually progressed more frequently to proteinuria than those who were not treated.30
CONCISE METHODS
Joslin Cohort
The Joslin Clinic is a large center for the treatment of patients of all ages with type 1 or type 2 diabetes. Clinic care includes control of diabetes as well as treatment of late diabetic complications. The clinic population is about 90% Caucasian and includes all social strata. The majority of patients reside in eastern Massachusetts and come to the clinic within 5 years of diagnosis. Many remain with the clinic for decades receiving integrated care of endocrinologists, nephrologists, and ophthalmologists. About one-fourth of the 16,000 patients under the care of the clinic have T1D (defined below). We screened a random half of this population in 1991 to 1992 for albuminuria as part of the Joslin Study on the Natural History of Microalbuminuria.12 Albuminuria was categorized into normo-, micro-, or macroalbuminuria on the basis of multiple measurements of the urinary ACR in random urines (see below for ACR criteria for defining macroalbuminuria). Patients diagnosed with macroalbuminuria at entry and additional patients with macroalbuminuria detected by systematic rescreening of the random half of the Joslin population during follow-up until 2000 were recruited into our study of the genetics of kidney disease in T1D. Between 2001 and 2004, we recruited all of the adult patients with T1D and macroalbuminuria attending the clinic. These patients came in majority from the part of the T1D Joslin population, which did not participate in the study in 1991 to 1992. Our institutional review board approved the protocol and consent procedures for this study.
Diagnosis of T1D and Macroalbuminuria
T1D was diagnosed if medical record review indicated that hyperglycemia was diagnosed before age 40 years and uninterrupted insulin treatment began within one year. The computer databases of the Joslin Clinic retain all of the measurements of urinary albumin, creatinine, and their ratio (ACR) obtained for research or clinical use since 1990 and provided the basis for diagnosing macroalbuminuria in clinic patients. Methods for measuring ACR and defining macroalbuminuria were described previously.12,31 Briefly, persistent macroalbuminuria was diagnosed when two out of three consecutive urine samples had an ACR (in mg/g of creatinine) ≥250 for men or ≥355 for women. The concentration of urinary creatinine is lower in women than men (on average); hence the ratio is higher for an equivalent albumin excretion rate. ACRs were calibrated to timed measurements of the albumin excretion rate, and these sex-specific ACR criteria are both equivalent to an albumin excretion rate of 300 μg/min, which we used as the lower bound for macroalbuminuria. All of the patients eligible for the study were approached and invited to participate (17% declined, with a similar proportion in each subcohort). Among the examined patients, review of medical records for evidence of nondiabetic renal disease excluded five patients because of ambiguous etiology. The total number of patients examined and considered for studies was 467. Out of this group 423 (91%) patients defined themselves as white, and they were included in this study.
Other Baseline Characteristics
Enrollment examinations were performed by trained recruiters and included a structured interview about medical history, BP measurements, and anthropometric measurements. Blood (nonfasting) was collected for biochemical studies and urine for ACR determination. In 2009 creatinine was measured in stored baseline serum specimens at the University of Minnesota with the Roche enzymatic assay (product number 11775685) on a Roche/Hitache Mod P analyzer. This method is calibrated to be traceable to an isotope dilution mass spectrometry (IDMS) creatinine reference method, and the calibration has been verified by measuring National Institutes of Standards and Technology Standard Reference Material No. 967. The IDMS-traceable Modified Diet in Renal Disease study formula (http://nkdep.nih.gov/labprofessionals/estimating_gfr.htm) and serum creatinine were used to determine estimated glomerular filtration rates (eGFRs). Staging of renal function was based on the National Kidney Foundation Chronic Kidney Disease staging criteria.32
In addition to using historical ACRs to diagnose macroalbuminuria, the ACR measured on the day of enrollment was used to characterize the intensity of urinary albumin excretion. Similarly glycemic control was assessed by the hemoglobin A1c on the day of enrollment.12 In 2009, Serum concentrations of cholesterol (total and HDL) in the enrollment samples were measured at the Joslin Clinical Laboratory using the Vitros 5.1 Chemistry System (Ortho Clinical Diagnostics).
The medical history questionnaire requested a list all of current medications and asked specifically about lipid-lowering drugs and common ACE-I and ARBs. The latter were then classified into ACE-I, ARB, diuretic, or other anti-hypertensive therapies.
Determination of Onset of ESRD and Mortality
Patients enrolled were followed for the development of ESRD and death by matching the study roster against the United States Renal Data System (USRDS) and the National Death Index (NDI). In the United States, persons who develop ESRD are eligible for Medicare support, and the USRDS tracks requests to Medicare for renal replacement therapy. During the years of this study, the criteria for acceptance into this program and for priority for transplant have not changed for this age group with T1D. The onset of ESRD was recorded as the date of first renal transplant or renal dialysis. The most recent query took place in October 2009 and covered all incident cases of ESRD between 1991 and the end of 2007.
The NDI (www.cdc.gov/nchs/r&d/ndi/what_is_ndi.htm) is a central index of death records on file in the vital statistics offices of the U.S. In addition to information from the NDI, the vital status and the circumstances surrounding death were abstracted from medical records. The cause of death was coded by ICD-9 and ICD-10 (depending on date of death) provided by the NDI database. Additional data were extracted from the USRDS data and from death certificates. Matching with the NDI is complete through the end of 2007.
Living subjects who were free of ESRD were contacted for follow-up in 2008 or 2009. We were able to ascertain the status (alive, ESRD, or death) of 98% of the subjects through 2008. We examined those without these events in the same fashion as their entry examination. Observation time was censored at the end of 2008.
Medical records of patients who died before diagnosis of ESRD were reviewed. Seven were reclassified as having reached ESRD before dying on the basis of the last eGFR before death and other information recorded at death. Additionally, three patients had stage 5 CKD at the last clinic visit, 1 to 3 years before 2008, but refused examination in 2008. We classified these patients as reaching ESRD, although this was not yet confirmed in USRDS. Deaths were assigned to cardiovascular causes on the basis of codes recorded in the National Death Index. For deaths before 1999, they were ICD-9 codes 401 to 450; for deaths after 1998, they were ICD-10 codes I10-I74.
The predicted number of deaths unrelated to ESRD was estimated using sex-, age-, and race-specific death rates in the U.S. population in 2007 (Center for Disease Control, National Vital Statistics Report, vol. 58 no. 19, Table 5).
Statistical Analyses
Descriptive characteristics of participants in this study were summarized using means and SD or medians and quartiles. Multidecrement life tables were used to estimate the cumulative risk of the two competing outcomes: ESRD or death.33
Covariate effects on time to events were first evaluated individually in Cox regression models. The subset with a significant P value of <0.05 was analyzed in a multivariate Cox regression model to remove redundant information. Characteristics eliminated in univariate analysis were reintroduced to determine whether they were significant after adjustment for other significant covariates (eGFR, ACR, and HbA1c). We considered quadratic and cubic representations of continuous covariates and tested for interactions between covariates using both their categorized and continuous representations, where applicable. We assessed proportionality of hazards with log-log survivor plots and by introducing interactions with time variable categorized into 2-year intervals within the 6-year follow-up, common for all three subcohorts. Analyses were carried-out in SAS for Windows, version 9.1.3 (SAS Institute, Cary, NC).
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This study was inspired by Dr. Diego Cantarovich of Nantes, France, who asked us for data to design the Pancreas Allotransplantation for Diabetic Nephropathy and Mild Chronic Renal Failure Stage Study.
This project was supported through JDRF research grant 1-2008-1018 (A.S.K.) and National Institutes of Health grants DK41526 (A.S.K.) and DK067638 (A.S.K.) and National Institutes of Health Child Health Services Research Training Program, grant HS00063 (E.T.R.), and JDRF fellowship grant 3-2009-397 (J.S.).
We thank Dr. Paul W. Eggers from NIDDK for help in matching the Joslin study roster against the USRDS.
The analysis and opinions expressed in this manuscript do not represent position of the NIDDK or USRDS.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Competing-Risk Analysis of ESRD and Death among Patients with Type 1 Diabetes and Macroalbuminuria,” on pages 537–544.
REFERENCES
- 1. Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T: Diabetic nephropathy in type 1 (insulin-dependent) diabetes: An epidemiological study. Diabetologia 25: 496–501, 1983 [DOI] [PubMed] [Google Scholar]
- 2. Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR: The changing natural history of nephropathy in type 1 diabetes. Am J Med 78: 785–794, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Borch-Johnsen K, Kreiner S: Proteinuria: Value as predictor of cardiovascular mortality in insulin-dependent diabetes mellitus. BMJ 294: 1651–1654, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mogensen CE: Long-term antihypertensive treatment inhibiting progression of DN. BMJ 285: 685–688, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parving HH, Andersen AR, Smidt UM, Svendsen PA: Early aggressive antihypertensive treatment reduces rate of decline in kidney function in DN. Lancet 1: 1175–1179, 1983 [DOI] [PubMed] [Google Scholar]
- 6. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy. The Collaborate Study Group. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Laffel LM, McGill JB, Gans DJ: The beneficial effect of angiotensin-converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group. Am J Med 99: 497–504, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D: Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605–612, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Limaye S, O'Kelly P, Harmon G, O'Neill D, Dorman AM, Walshe J, Donohoe J, Little D, Conlon PJ, Keogan MT: Improved graft survival in highly sensitized patients undergoing renal transplantation after the introduction of a clinically validated flow cytometry crossmatch. Transplantation 87: 1052–1056, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Mange KC, Joffe MM, Feldman HI: Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 344: 726–731, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Mange KC, Weir MR: Preemptive renal transplantation: Why not? Am J Transplant 3: 1336–1340, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH: Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med 332(19): 1251–1255, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Forsblom C, Harjutsalo V, Thorn LM, Wadén J, Tolonen N, Saraheimo M, Gordin D, Moran JL, Thomas MC, Groop P-H. on behalf of the FinnDiane Study Group: Competing-risk analysis of ESRD and death among patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol 22: 537–544, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jorsal A, Tarnow L, Flyvbjerg A, Parving HH, Rossing P, Rasmussen LM: Plasma osteoprotegerin levels predict cardiovascular and all-cause mortality and deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetologia 51: 2100–2107, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Parving HH, Jacobsen P, Rossing K, Smidt UM, Hommel E, Rossing P: Benefits of long-term antihypertensive treatment on prognosis on diabetic nephropathy. Kidney Int 49: 1778–1782, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Astrup AS, Ttarnow L, Rossing P, Pietraszek L, Hansen PR, Parving HH: Improved prognosis in type 1 diabetic patients with nephropathy: A prospective follow-up study. Kidney Int 68: 1250–1257, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Appel LJ, Wright JT, Jr, Greene T, Kusek JW, Lewis JB, Wang X, Lipkowitz MS, Norris KC, Bakris GL, Rahman M, Contreras G, Rostand SG, Kopple JD, Gabbai FB, Schulman GI, Gassman JJ, Charleston J, Agodoa LY: Long-term effects of rennin-angiotensin system-blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African Americans. Arch Intern Med 168: 832–839, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, UKPDS Group: Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study. Kidney Int 63: 225–232, 2003 [DOI] [PubMed] [Google Scholar]
- 19. DCCT/EDIC Research Group: Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: The Epidemiology of Diabetes Intervention and Complication (EDIC) study. JAMA 290: 2159–2167, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cantarovich D: Pancreas transplant alone, proposal of a new study: PANCREAStudy (Pancrease Allotransplanantion No Chronic Renal Ailure Study). Annual meeting of Euro. Soc. Organ Tansplant September 2007, Praha, Czech Republic [Google Scholar]
- 21. Fioretto P, Steffes MW, Southerland DE, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Ingelfinger JR: Aliskiren and dual therapy in type 2 diabetes mellitus. N Engl J Med 358: 2503–2505, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK: Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 258: 2433–2446, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Tuttle KR: Protein kinase C-beta inhibition for diabetic kidney disease. Diabetes Res Clin Pract 82[Suppl 1]: S70–S74, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Williams ME, Bolton WK, Khalifah RG, Degenhardt TP, Schotzinger RJ, McGill JB: Effects of pyrioxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am J Nephrol 27: 605–614, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Deelman L, Sharma K: Mechanisms of kidney fibrosis and the role of antifibrotic therapies. Curr Opin Nephr Hypertension 18: 85–90, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Brigstock DR: Strategies for blocking the fibrogenic actions of connective tissue growth factor (CCN2): From pharmacological inhibition in vitro to targeted siRNA therapy in vivo. J Cell Commun Signal 3: 5–18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones E, Pu H, Kyprianou N: Targeting TGF-beta in prostate cancer: Therapeutic possibilities during tumor progression. Expert Opin Ther Targets 13: 227–234, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Becker BN, Rush SH, Dykstra DM, Becker YT, Port FK: Preemptive transplantation for patients with diabetes-related kidney disease. Arch Intern Med 166: 44–48, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Ficociello LH, Perkins BH, Silva KH, Finkelstein DM, Ignatowska-Switalska H, Gaciong Z, Cupples LA, Aschengrau A, Warram JH, Krolewski AS: Determinants of progression from microalbuminuria to proteinuria in type 1 diabetes patients treated with ACE Inhibitors. Clin J Am Soc Nephrol 2: 461–469, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Warram JH, Gearin G, Laffel L, Krolewski AS: Effect of duration of IDDM on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol 7: 930–937, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National Kidney Foundation. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Namboodiri K, Suchindran C: Life table techniques and their applications in studies in population, Orlando, Florida, Academic Press, 1987 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.