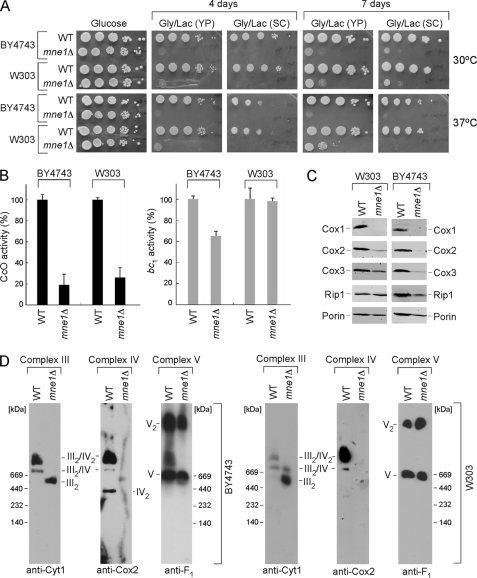
FIGURE 1.
Deletion of Mne1 results in a CcO-specific respiratory defect. A, respiratory growth of mne1Δ strains. Mutant and the isogenic wild-type (WT) cells were pre-grown in complete (YP) or synthetic (SC) liquid medium, serially diluted, and spotted onto the respective plates containing 2% glucose or glycerol/lactate as a carbon source. Pictures of the plates were taken after 4 and 7 days of incubation at 30 or 37 °C. B, CcO and SDH/bc1 activities from the mne1Δ and corresponding WT mitochondria, normalized to total protein. Enzymatic activities are shown as a percentage of wild-type specific activity, error bars indicate S.D. (n = 3). C, steady-state levels of core CcO subunits (Cox1, Cox2, and Cox3) and Rip1 were analyzed by immunoblotting 20 μg of mitochondria isolated from the indicated WT and mne1Δ strains. The mitochondrial outer membrane porin served as a loading control. D, BN-PAGE analysis of the respiratory complexes in WT and mne1Δ mitochondria. Isolated organelles (75 μg) were solubilized in a lysis buffer containing 1% digitonin. Protein complexes were separated on the continuous 5–13% gradient gel under native conditions. Western blotting with antibodies against Cox2, Cyt1, and Atp2 (anti-F1) was used to assess the distribution of respiratory complexes.