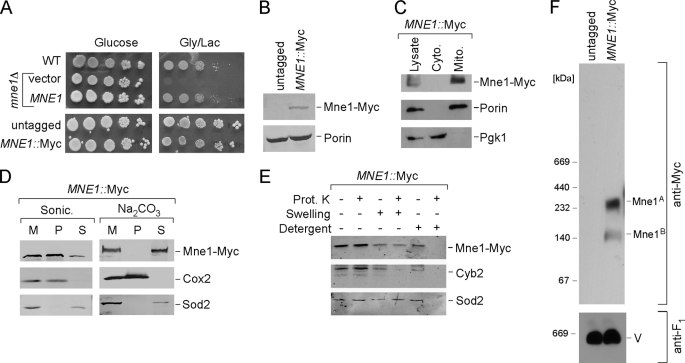
FIGURE 2.
Mne1 is a soluble matrix protein associated with mitochondrial inner membrane. A, respiratory growth of mne1Δ cells complemented with the vector-borne MNE1 and cells with chromosomally tagged Mne1. Cells were grown and tested as in E. B, whole cell lysates of untagged and MNE1::Myc strains were analyzed by SDS-PAGE and Western blot with anti-Myc and anti-porin. C, immunoblot of mitochondria (Mito.) and the post-mitochondrial fraction (Cyto.) purified from the MNE1::Myc strain. Anti-Myc antibodies have been used to detect tagged Mne1. Phosphoglycerol kinase (Pgk1), a cytosolic marker and Porin, a mitochondrial outer membrane protein were used to verify fractionations. D, isolated mitochondria (50 μg) were sonicated in 20 mm HEPES (pH 7.4) and 50 mm NaCl or incubated on ice for 30 min with 0.1 m sodium bicarbonate (pH 11.5). 2 mm phenylmethylsulfonyl fluoride was added in both cases. The soluble and pellet fractions were separated by centrifugation at 165,000 × g for 1 h, and analyzed by Western blot. Soluble matrix protein Sod2 and integral membrane protein Cox2 were detected with the respective antibodies. M, total mitochondria; S, supernatant; P, pellet. E, intact or osmotically swollen mitochondria were incubated with or without 0.1 mg/ml of proteinase K (Prot. K) for 30 min on ice. A combination of detergent and proteinase K treatment was used to exclude the possibility that Mne1-Myc is a proteinase-resistant protein. Following centrifugation, the treated organelles were separated by SDS-PAGE and analyzed by immunoblotting with anti-Myc, anti-Cyb2, and anti-Sod2. F, mitochondria (50 μg) isolated from untagged or MNE1::Myc cells were solubilized with 1% digitonin and analyzed by BN-PAGE as described in the legend to Fig. 1D.