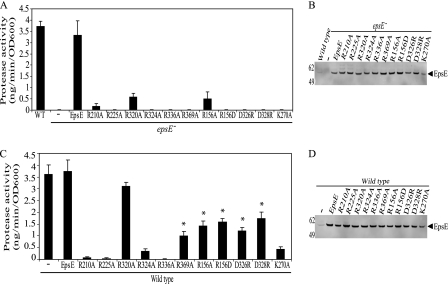
FIGURE 2.
EpsE point mutations inhibit protease secretion in vivo. epsE− (A and B) or wild type (C and D) strains of V. cholerae containing pMMB67 vector, pMMB EpsE, or the plasmid-encoded EpsE with the following point mutations, R210A, R225A, R320A, R324A, R336A, R369A, R156A, R156D, D326R, D328R, or K270A, were grown overnight in LB + 10 μm isopropyl-1-thio-β-d-galactopyranoside. Culture supernatants and cells were separated by centrifugation. A and C, culture supernatants were analyzed for the presence of extracellular protease activity using a fluorescence-based assay with peptide-conjugated methylcoumarin as a substrate. The amount of methylcoumarin liberated was compared with a standard curve with each sample assayed at least three independent times. The results are presented as nanogram of methylcoumarin hydrolyzed per minute, normalized to the A600 of the culture ± S.E. * indicates a statistically significant difference (p < 0.05) when compared with both wild type and EpsE-K270A. OD, optical density. B and D, cell extracts were subjected to SDS-PAGE and immunoblotted with anti-EpsE. Molecular mass markers are indicated on the left, and the position of EpsE is indicated by the arrow.