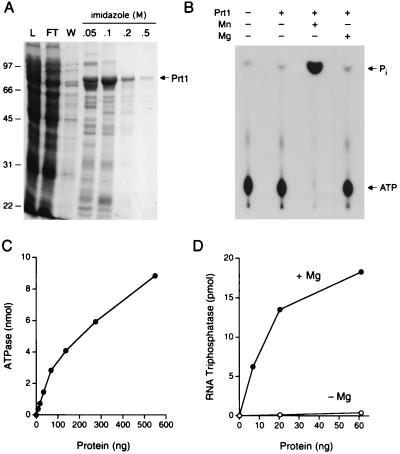
Figure 5.
Characterization of P. falciparum RNA triphosphatase. (A) Prt1 purification. Aliquots (15 μl) of the soluble bacterial lysate (L), the Ni-agarose flow-through (FT), wash (W), and indicated imidazole eluates were analyzed by SDS-PAGE. The fixed gel was stained with Coomassie brilliant blue dye. (B) Manganese-dependent NTP hydrolysis. ATPase reaction mixtures contained 0.6 μg of Prt1 and divalent cations as specified. The reaction products were analyzed by TLC and visualized by autoradiography. The positions of [γ-32P]ATP and 32Pi are indicated. (C) Prt1 titration. ATPase reaction mixtures contained 2 mM MnCl2 and Prt1 as specified. (D) RNA triphosphatase activity. Reaction mixtures contained 2 μM γ-32P-labeled poly(A), either 2 mM MgCl2 or no added divalent cation, and recombinant Prt1 as specified.