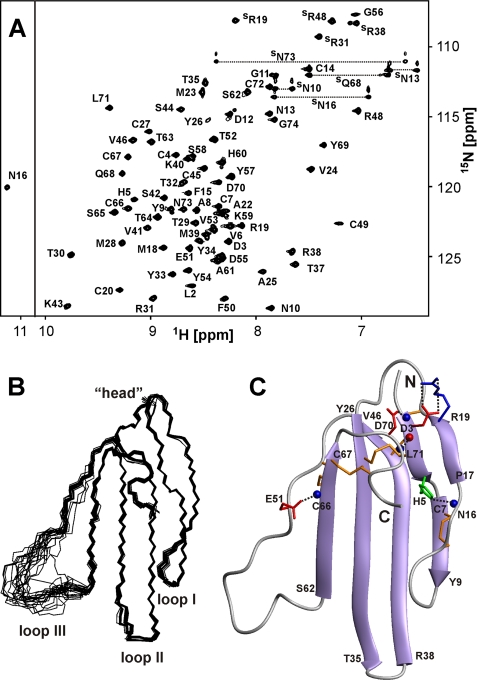
FIGURE 2.
NMR data define the spatial structure of ws-LYNX1 in aqueous solution. A, two-dimensional 1H,15N-HSQC spectrum of ws-LYNX1 (0.5 mm, pH 5.3, 25 °C). The obtained resonance assignments are shown. The resonances of side chain groups are marked with an S. The resonances of Asn and Gln NH2 groups are connected by dotted lines. B, the set of the best 20 ws-LYNX1 structures superimposed over the backbone atoms in regions with well defined structure. The three loops and head of the protein are labeled. C, ribbon representation of ws-LYNX1 spatial structure. The electrostatic and hydrogen bonding interactions that stabilize the protein fold are shown. The Arg, Asp/Glu, and His side chains participating in salt bridges and hydrogen bonds with backbone amides are in blue, red, and green, respectively. The hydrogen bond HN Leu71–CO Asp3 is also shown. Backbone amide and carbonyl groups are shown by blue and red spheres, respectively. The disulfide bonds are shown in orange.