FIGURE 1.
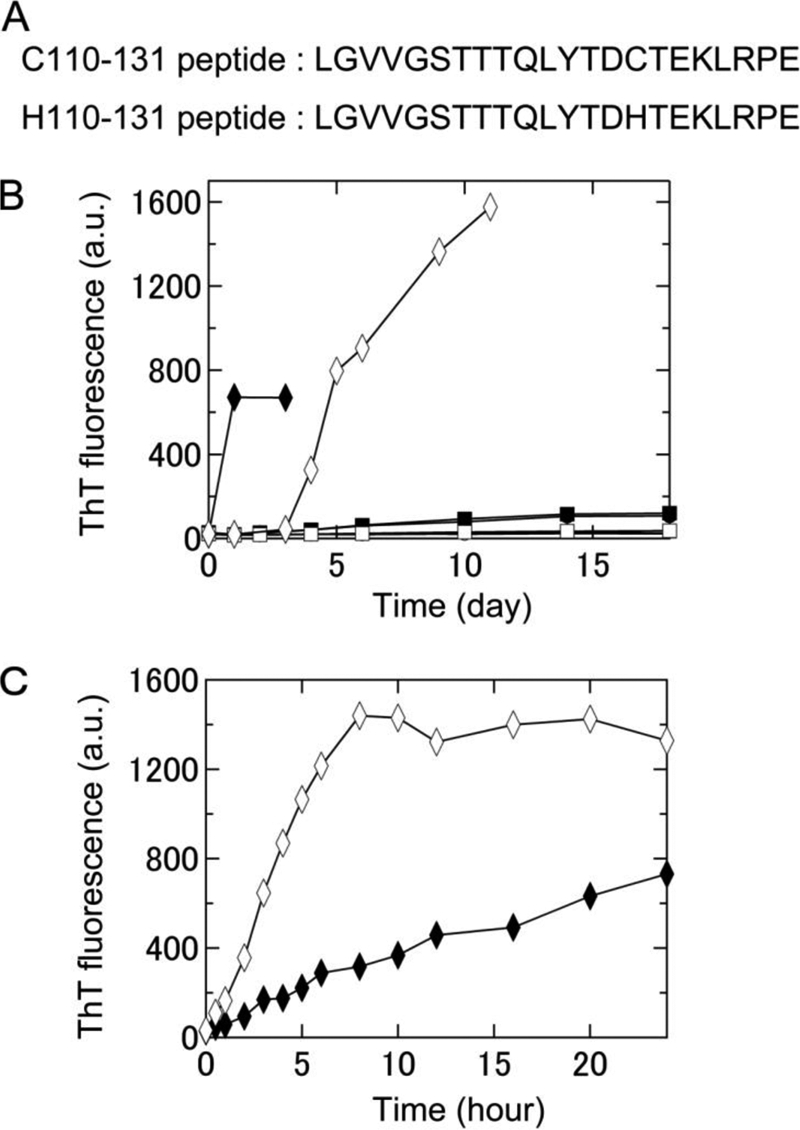
Formation of amyloid fibrils of two keratoepithelin peptide fragments, C110–131 and H110–131, under various conditions at neutral pH monitored using ThT fluorescence. A, amino acid sequences of the C110–131 and H110–131 peptides. B, time course of the spontaneous formation of C110–131 (▴, ●, ■, ♦) and H110–131 (△, ○, □, ♢) fibrils in 67 mm sodium phosphate buffer (pH 7.4) at 4 °C (▴, △), 67 mm sodium phosphate buffer (pH 7.4) at 37 °C (●, ○), 50 mm sodium phosphate buffer (pH 7.0) containing 100 mm NaCl at 37 °C (■, □), or 50 mm sodium phosphate buffer (pH 7.0) containing 100 mm NaCl and 0.5 mm SDS at 37 °C (♦, ♢). C, time course of seed-dependent growth of C110–131 (♦) and H110–131 (♢) peptides in 50 mm sodium phosphate buffer (pH 7.0) containing 100 mm NaCl and 0.5 mm SDS at 37 °C. Concentration of seeds was 5 μg/ml.
