Abstract
Our recent animal and human studies revealed that chronic hyponatremia is a previously unrecognized cause of osteoporosis that is associated with increased osteoclast numbers in a rat model of the human disease of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). We used cellular and molecular approaches to demonstrate that sustained low extracellular sodium ion concentrations ([Na+]) directly stimulate osteoclastogenesis and resorptive activity and to explore the mechanisms underlying this effect. Assays on murine preosteoclastic RAW 264.7 cells and on primary bone marrow monocytes both indicated that lowering the medium [Na+] dose-dependently increased osteoclast formation and resorptive activity. Low [Na+], rather than low osmolality, triggered these effects. Chronic reduction of [Na+] dose-dependently decreased intracellular calcium without depleting endoplasmic reticulum calcium stores. Moreover, we found that reduction of [Na+] dose-dependently decreased cellular uptake of radiolabeled ascorbic acid, and reduction of ascorbic acid in the culture medium mimicked the osteoclastogenic effect of low [Na+]. We also detected downstream effects of reduced ascorbic acid uptake, namely evidence of hyponatremia-induced oxidative stress. This was manifested by increased intracellular free oxygen radical accumulation and proportional changes in protein expression and phosphorylation, as indicated by Western blot analysis from cellular extracts and by increased serum 8-hydroxy-2′-deoxyguanosine levels in vivo in rats. Our results therefore reveal novel sodium signaling mechanisms in osteoclasts that may serve to mobilize sodium from bone stores during prolonged hyponatremia, thereby leading to a resorptive osteoporosis in patients with SIADH.
Keywords: Ascorbic Acid, Bone, Differentiation, Macrophage, Oxidative Stress, Hyponatremia, Osteoclast, Osteoporosis, Sodium Sensing
Introduction
Hyponatremia, defined as serum sodium ion concentration ([Na+]) less than 135 mmol/liter, is a frequently encountered electrolyte abnormality in patients. Chronic hyponatremia is an especially common disorder in elderly people, often due to the dysregulation of hypothalamic osmoregulatory mechanisms, leading to the syndrome of inappropriate antidiuretic hormone secretion (SIADH).2 Hyponatremia may also arise from chronic heart failure or cirrhosis and from treatment with a large number of drugs, including diuretics and selective serotonin reuptake inhibitors. The estimated prevalence of chronic hyponatremia in the United States is in the range of 3.2–6.1 million persons annually, 75–80% of whom are without obvious neurological symptoms. Although chronic hyponatremia is often considered to be “asymptomatic,” recent reports have shown its adverse effects, namely impaired gait stability and neurocognitive functions and, therefore, a greater risk of falls and fractures. In one recent case-controlled study of asymptomatic chronic hyponatremic patients, even mild hyponatremia was associated with a 67-fold increased odds ratio for falling compared with normonatremic controls. Even more alarming, a recent study from Belgium found that mild asymptomatic hyponatremia was associated with bone fractures in ambulatory elderly subjects (adjusted odds ratio of 4.16, 95% confidence interval: 2.24–7.71) (1), and the incidence of hyponatremia in patients aged 65 or older with skeletal fractures was more than double that of patients with no fracture (9.1 and 4.1%, respectively; p = 0.007) (2). Recent studies from our laboratory have indicated that hyponatremia is also associated with osteoporosis due to increased osteoclastic bone resorption in a rat model of SIADH (3). Histomorphometric analysis indicated that osteoclast number was increased 5–10-fold in excised femurs, tibia, and spine from hyponatremic rats, and analysis of blood samples revealed no significant metabolic or hormonal change that could account for the increased osteoclastic bone resorption (3).
Early studies of radionucleotide distribution indicated that approximately one-third of the body's sodium is stored in the bone matrix along with calcium and phosphorus and is released during osteoclastic resorption (4). A more recent study has also shown that large amounts of sodium are stored in an osmotically inactive compartment in the bones of dogs and are released from this store during prolonged dietary sodium deprivation (5). The primary components of bone matrix are removed by osteoclasts first by demineralization of the inorganic mineral through acidification of the local bone microenvironment, followed by degradation of collagen by the cysteine protease cathepsin K. Although the mechanisms that initiate osteoclastogenesis and osteoclastic bone resorption in response to hyponatremia have not been investigated, activation of osteoclastic bone resorption in response to calcium deprivation and various other stimuli has been studied extensively (reviewed in Refs. 6 and 7). Stimulation of osteoclastogenesis and osteoclast activity is known to be associated with sex steroid deficiency-related osteoporosis, metastasis-induced osteolysis, Paget disease, rheumatoid arthritis, and periodontal disease. Osteoclasts arise from hematopoietic precursors in the monocyte-macrophage lineage from the blood or the bone marrow. Cytokines and bone-resorbing agents act on osteoclast lineage cells to promote osteoclastogenesis through a common mechanism that requires the receptor activator of NF-κB (RANK) ligand (RANKL) as the essential mediator of osteoclast formation in response to all known stimuli. Osteoblasts/stromal cells are also the source of macrophage colony-stimulating factor (M-CSF), which plays a crucial role in osteoclast formation by promoting the proliferation of osteoclast precursors. Osteoclastogenesis also requires exposure to calcitriol, the active form of vitamin D. In addition, cytokine and tyrosine kinase receptors on osteoclasts (6), as well as oxidative stress (8), can modify RANK signaling. Bone resorption is also regulated by G-protein-coupled receptors, estrogen receptors, integrin, and calcium-mediated signals operating via the nitric oxide signaling pathway (6).
Mechanisms underlying osmoregulation and sodium sensing are best understood in the hypothalamus, but these are capabilities essential for the survival of all eukaryotic cells as well. Release of the antidiuretic hormone arginine vasopressin (AVP) in response to increases in osmolality is controlled by specific and highly sensitive osmoreceptors in the hypothalamus (9, 10). Hypoosmolar conditions trigger an adaptation to maintain cell volume by extruding intracellular solutes, including electrolytes and organic osmolytes, in cells of the brain and peripheral tissues. This adaptation is accomplished by stretch-activated, voltage-gated potassium channels and swelling-activated chloride channels. The TRPV4 (transient receptor potential vanilloid type 4) channel is postulated to comprise an important element of the central tonicity-sensing mechanism in the mammalian hypothalamus and is activated by hypotonic stress in vitro (11). Sensing of the intracellular sodium concentration and the responses to rising intracellular sodium concentrations is largely accomplished by the activity of the plasma membrane Na+/K+-ATPase and its associated proteins, such as the sucrose nonfermenting-1-related serine/threonine kinase, SIK1 kinase (12). The molecular mechanism by which low extracellular [Na+] is detected is not yet understood. High [Na+] has been reported to inhibit the activity of the epithelial Na+ channel, a phenomenon of self-inhibition of epithelial sodium transport in the distal nephron, distal colon, and airways (13). The calcium-sensing receptor and the cystic fibrosis transmembrane conductance regulator are also involved in the adaptation to high salinity in fish (14).
The experiments in this paper provide support for a direct effect of low extracellular sodium concentration to stimulate osteoclastogenesis and osteoclastic resorption without activation of signaling through osteoblasts, a key mechanism that is unique to hyponatremia-induced osteoporosis. By manipulating culture media during osteoclastogenesis from RAW 264.7 murine monocytic preosteoclastic cells, we studied whether low sodium or low osmolality or both were responsible for activating osteoclast differentiation and bone resorbing activity. We then explored the potential mechanisms underlying activation of osteoclastogenesis and osteoclastic resorption by low extracellular sodium concentrations. Our findings indicate the involvement of extracellular [Na+] in regulating ascorbic acid uptake and providing protection from oxidative stress. Importantly, this mechanism is similar in many ways to the mechanisms through which estrogen and androgen deficiency activate osteoclasts (15).
EXPERIMENTAL PROCEDURES
Cell Culture
Low passage number murine monocytic RAW 264.7 cells were obtained from the American Type Culture Collection (Manassas, VA). These cells can differentiate into mature osteoclasts upon stimulation with RANKL and M-CSF. Cells were grown in minimal essential medium α (4.5 g/liter glucose; Invitrogen). For selected experiments, we used minimal essential medium α without ascorbic acid (custom-produced by Invitrogen) or the same medium with the addition of 50 mg/liter l-ascorbic acid. To generate media with the lower, desired [Na+] of 131, 129, 121, and 112 mmol/liter, while maintaining other parameters identical to the normal sodium medium, we added a mixture of distilled water, glucose, minerals (except sodium), amino acid concentrate, and vitamins (Mediatech Inc., Herndon, VA) in appropriate amounts. When adjustment of osmolality was needed, mannitol was added to each low [Na+] medium to reach 290 mosmol/kg H2O. Every medium was supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT), 2 mm glutamine, and 1% penicillin/streptomycin (Invitrogen) and was filter-sterilized. Sodium concentration and osmolality of media were measured before application to cells using a sodium analyzer (Coulter ELISE, Beckman, Fullerton, CA) and a freezing point osmometer (model 3900, Advanced Instruments, Inc., Norwood, MA). To adapt cells to low sodium concentrations, the extracellular sodium concentration was decreased gradually with daily medium changes, with every medium change to lower [Na+] by 4 mmol/liter, until the desired final concentration was reached. Adapted cells were grown in media with the target sodium concentration for at least 7 days before experiments. Cells were cultured at 37 °C in a 5% CO2 incubator.
Cell Viability Measurements
RAW 264.7 cells were adapted to and grown for 7 days at target [Na+] media and then plated to 96-well plates at 50,000 cells/well to grow for another 24 h at the same graded [Na+] media in triplicates. Viability and cytotoxicity were determined using the LIVE/DEAD® cell viability kit (Invitrogen) according to manufacturer's instructions. Calcein fluorescence was measured using fluorescein optical filters and ethidium homodimer-1 fluorescence using rhodamine filters (PerkinElmer Life Sciences). Controls included digitonin-treated cells, cells without indicator, and plating escalating numbers of cells (from 100 to 100,000 cells/well).
Osteoclastogenesis Assays
Control and adapted RAW 264.7 cells were subcultured into 24-well plates (Costar) at 1 × 104 cells/well, with each well containing 1 ml of medium. The next day, media were changed to differentiation media generated with the addition of 10 ng/ml recombinant mouse M-CSF (Sigma-Aldrich) and 50 ng/ml recombinant RANKL (R&D Systems, Inc., Minneapolis, MN) to the medium with the appropriate [Na+]. Although RAW 264.7 cells form tartrate-resistant acid phosphatase (TRAP+)-stained multinucleated cells with RANKL alone, without M-CSF (16), we and others (17) found that these cells did not resorb dentin to the same extent as cells differentiated with both RANKL and M-CSF (data not shown). Sodium concentrations were maintained during the differentiation as indicated. Differentiation media were changed on alternate days for 3–14 days for time course studies and for 7 days in every other experiment. At the termination of the experiments, cells were fixed for 10 min in 3.7% formalin in citrate buffer prepared with 65% acetone, pH 5.8, and stained using the acid phosphatase kit and protocol from Sigma-Aldrich. Each experiment was done in triplicate and repeated at least three times.
Osteoclastogenesis was also evaluated in two experiments from primary rat bone marrow monocyte (BMM) cultures, as described previously (18, 19) with modifications. All procedures concerning animal experiments were performed according to the Guidelines for the Care and Use of Animals available on the National Academies Press Web site. The Institutional Animal Care and Use Committee of Georgetown University approved all animal experimentation according to federal and institutional policies and regulations. For the first experiment, unfractionated bone cells were prepared from the marrow of femora from four 11-month-old male Sprague-Dawley rats (Taconic Farms Inc., Hudson, NY) and disaggregated in minimal essential medium α supplemented with 10% FBS, Glutamax, and penicillin/streptomycin (Invitrogen). Unfractionated cells were washed three times by pelleting and resuspension in fresh medium and then plated onto eight 10-cm culture dishes (Fisher) and cultured overnight in the same medium supplemented with 0.2 ng/ml M-CSF (R&D Systems, Minneapolis, MN). Nonadherent cells were collected, and mononuclear cells were recovered from the interface of Ficoll-Paque Premium gradients (GE Healthcare Life Sciences). BMMs were resuspended at a density of 5 × 104 cells/ml in fresh medium supplemented with both 0.2 ng/ml M-CSF and 0.07 ng/ml RANKL (R&D Systems, 462 TEC/CF) and placed into 6-well plates. Over the next 5 days, cells were adapted to grow in the same medium while gradually lowering the [Na+] to reach final [Na+] of 136, 131, 126, 121, and 117 mmol/liter. Cells were then either allowed to continue to differentiate in the 6-well plates for 14 days or scraped and transferred to 24-well plates with dentin slices for the resorption assay. At the end of differentiation, cells were fixed and stained for TRAP.
In the second experiment, BMMs were prepared from seven normonatremic and seven hyponatremic 11-month-old male Sprague-Dawley rats. To generate hyponatremic rats, animals were fed a liquid diet for 2 weeks and were infused with the vasopressin V2R agonist desmopressin (dDAVP, Aventis) via subcutaneously implanted minipumps (Alzet model 2004, Durect Co., Cupertino, CA) releasing dDAVP at a rate of 0.25 μl/h (5 ng/h). This infusion of dDAVP alone does not produce hyponatremia in the absence of water loading. The hyponatremic rats received an ad libitum (average 28 ml/day) liquid diet (F5400sp, Bio-Serv) with a caloric density of 1.6 kcal/ml (72 kcal/day/rat), vitamin D3 content of 3.2 IU/ml (144 IU/day/rat), and calcium content of 2 mg/ml (90 mg/day/rat). The normonatremic animals were pair-fed with the same liquid diet, adjusting the volume every day, and received buffer-containing pumps without dDAVP. Hyponatremia ([Na+] = 117 ± 3 mmol/liter) was maintained for 2 weeks before sacrifice and collection of BMMs. To differentiate osteoclasts from BMMs of hyponatremic rats, all medium [Na+] values were lowered, while maintaining the other medium components the same as described for the first experiment. Briefly, unfractionated cells were cultured for 24 h in media with M-CSF, and then mononuclear cells were separated with a Ficoll gradient. Cells were cultured in differentiation media with M-CSF and RANKL for 11 days, with half of the media changed on alternate days. TRAP-positive multinuclear cells were counted from a 10-mm2 area, and colonies formed on 10-cm culture dishes were scored using a microscope.
Bone Resorption Assays
Control and adapted cells were subcultured into 24-well resorption plates coated with calcium hydroxyapatite (OAAS, Osteogenic Core Technologies USA, Irvine, CA) at 1 × 104 cells/well, with 1 ml of differentiation media as in the osteoclastogenesis assays. Differentiation media were changed on alternate days for 14 days. In one set of experiments, fully differentiated osteoclasts (after 7 days in differentiation media with either normal or low [Na+]) were subcultured into the OAAS wells at 4,000 cells/well and cultured for an additional 7 days in the same differentiation media. At the termination of the resorption experiments, cells were removed by 1 n sodium hydroxide, and the remaining calcium hydroxyapatite was stained using 5% silver nitrate under intense light exposure. Each experiment was done in triplicate and repeated at least three times.
Sperm whale dentin slices (300 μm thick, 4-mm diameter; generously provided by Dr. David Roodman (University of Pittsburgh School of Medicine, Pittsburgh, PA)) were sterilized overnight in 70% ethanol, air-dried, and presoaked in media containing the appropriate target [Na+] for 24 h and then placed into 12-well plates. RAW 264.7 cells adapted to grow in media with reduced [Na+] as well as BMMs adapted to differentiate in normal and reduced [Na+] media with M-CSF and RANKL were seeded into 12-well plates with the dentin discs (1 × 105 cells/well) and cultured for 14 days. Half of the differentiation media were replaced with fresh media on alternate days. Discs were removed, rubbed between fingers, washed, soaked in 500 μl of CytoBusterTM solutions (Novagen, EMD Chemicals Inc. San Diego, CA) to remove cells and debris, stained for 3.5 min in 1% toluidine blue solution, and then washed in H2O five times. The remaining osteoclasts in the wells were fixed and stained for TRAP.
Microscopy
The number of TRAP-positive multinucleated cells containing three or more nuclei was determined per mm2 of culture area using a Zeiss 410 inverted microscope fitted with a motorized stage and a Sony color camera. 25 images were collected from triplicate wells each, using the automated imaging system driven by software (Osteo II) from BioQuant Image Analysis Corp. (Nashville, TN). Resorption areas (unstained areas) per mm2 were determined from 25 images/well by automated gray scale segmentation, using the same imaging system and software. Resorption areas from the dentin slices were measured from the entire area.
Fluorescence Measurement of Cytosolic Free [Ca2+]
RAW 264.7 cells were first adapted to grow in reduced sodium media for 7 days as described under “Cell Culture.” The day before experiments, 5 × 106 cells were plated into 6-well plates in duplicates from each Na+ concentration. The next day, wells were washed three times in appropriate Na+-containing buffer A (0.1 mg/ml bovine serum albumin, 20 mm HEPES in Hanks' balanced salt solution that was generated from components with NaCl content adjusted to reach final [Na+] of 136, 131, 126, 121, and 117 mmol/liter) while maintaining other components identical and then incubated with loading buffer containing the fluorescent calcium indicator Fluo-4/AM (Invitrogen; 4 μm in DMSO), pluronic F-127 (20% (w/v) in DMSO), PowerLoadTM concentrate (Invitrogen), and normal or appropriately reduced [Na+] buffer A). After a 30-min incubation with the indicator solution at 37 °C, cells were washed in indicator-free normal or reduced [Na+] buffer A, harvested by scraping, and resuspended in 1 ml of the same indicator-free buffer A to reach 106 cells/ml, and 1-ml aliquots of loaded cell solutions were transferred to disposable polystyrene microcuvettes containing a spin bar and incubated for 30 min at room temperature. Fluorescence was measured with a spectrofluorometer with continuous mixing (Photon Technology International, Birmingham, NJ) (monochromator-based system with Felix software), using 494 ± 2-nm excitation and 516 ± 2-nm emission fluorescence. Measurements were taken from duplicate samples of each [Na+] before and immediately after the addition of thapsigargin (Tocris Bioscience, Ellsville, MO) (50 μm in DMSO). To measure [Ca2+] in the presence of serum, normal and reduced [Na+]-adapted cells were plated into a 96-well dish at 200,000 cells/well in triplicates for overnight. Indicator loading and fluorescence measurements were with the Fluo-4 NW DirectTM calcium assay kit (Invitrogen) according to the manufacturer's instructions, except the assay buffer was replaced with reduced [Na+] assay buffer when indicated, and fluorescence was measured with a 96-well plate reader with fluorescein filters (Wallac Victor reader). For both assays, calcium calibration buffer dilutions with Fluo-4 pentapotassium salt were used for calibration according to instructions from Invitrogen.
Measurement of Ascorbic Acid Uptake
RAW 264.7 cells were adapted to grow in media with the desired [Na+], as indicated (139, 131, 124, and 112 mmol/liter). These medium [Na+] values were maintained throughout each experiment. Cells were subcultured into 12-well plates, and the next day, the media were changed to FBS-free media three times 15 min apart and then incubated in 250 μl of FBS-free media with 1 μCi of l-[1-14C]ascorbic acid (specific activity 8.2 mCi/mmol; PerkinElmer Life Sciences) for 60 min. After removal of the isotope, the reaction was terminated by repeatedly adding (three times) 5 volumes of ice-cold Ca2+- and Mg2+-free [Na+] adjusted buffers with 10 mmol/liter unlabeled ascorbic acid. Cells were solubilized in 10 mmol/liter Tris-HCl (pH 8.0) containing 0.2% SDS, and the incorporated radioactivity was determined by liquid scintillation counting. Values were corrected for protein content. Each experiment was done in triplicate and repeated three times.
Measurement of Reactive Oxygen Species (ROS) Generation
RAW 264.7 cells grown in media with the indicated [Na+] were subcultured into 96-well plates and incubated with 25 μm 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCF) (Invitrogen) with and without 300 μm hydrogen peroxide for 30 min and with 1 μm Hoescht 33342 dye for the final 5 min (Image-ITTM LIVE Green detection kit, Invitrogen). Fluorescence was measured using a fluorometer plate reader (Tecan US Inc., Durham, NC; model Ultra384), and DCF fluorescence values were corrected with values of Hoechst fluorescence (DNA content).
Western Blot Analysis
Whole cells lysates from RAW 264.7 cells, grown in media with the indicated Na+ concentrations, were generated using the CytoBusterTM protein extraction reagents from Novagen, Inc. (Madison, WI) supplemented with a CompleteTM protease inhibitor mixture tablet (Roche Applied Science) and HALTTM phosphatase inhibitor solution (Thermo Scientific Pierce). Protein concentrations were determined using the Coomassie Plus kit (Pierce). Samples were denatured, and equal amounts of protein (5 μg for β-catenin and β-actin; 30 μg for everything else) were separated by 10% SDS-polyacrylamide gel electrophoresis. Proteins were then transferred to nitrocellulose membranes, and membranes were blocked and probed with overnight incubation at 4 °C with either of the following primary antibodies: mouse monoclonal against Akt and phosphorylated Akt (Ser473) (Cell Signaling, Technology, Danvers, MA; 1:1000 dilution for both), mouse monoclonal against p53 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; 1:5000), polyclonal rabbit against TNF-α (PeproTech, Rocky Hill, NJ; 1:1000 dilution), mouse monoclonal against β-catenin (BD Transduction Laboratories, San Jose, CA; 1:500 dilution), polyclonal rabbit against native p66Shc (BD Biosciences Transduction Laboratories, 1:1000 dilution), rabbit polyclonal against phosphorylated p66Shc (Tyr317) (Millipore/Upstate, Billerica, MA; 1:2,000 dilution), monoclonal rabbit against total NF-κB p65 and against phospho-NF-κB p65 (Ser536) (Cell Signaling Technologies; 1:1,000 dilution for both), rabbit polyclonal against c-Myc (Sigma-Aldrich; 1:1000 dilution), mouse monoclonal against JNK (Pierce; 1:1,000 dilution). We used β-actin as an internal standard for protein loading (antibody from Sigma-Aldrich; 1:5,000 dilution). Membranes were incubated with goat anti-mouse (Santa Cruz Biotechnology, Inc.; 1:5,000 dilution) or anti-rabbit (Pierce) HRP-conjugated secondary antibodies for 1 h, washed, and developed using the enhanced chemiluminescence detection kit from Pierce. Densitometry of band intensities was performed using GeneTools (Syngene, Frederick, MD). Western blot analyses for each protein were done from three separate extracts.
In Vivo Oxidative Damage Marker
22-month-old female F344 Brown Norway hybrid rats weighting 200 ± 9 g were obtained from the NIA, National Institutes of Health, supplied by Harlan-Sprague-Dawley (Indianapolis, IN). Rats were infused with dDAVP via subcutaneously implanted minipumps at a rate of 0.25 μl/h (5 ng/h) and were fed the liquid diet described under “Osteoclastogenesis Assays.” The pumps were replaced monthly to maintain continuous antidiuresis. The normonatremic animals were pair-fed with the same liquid diet, adjusting the volume every day, and received buffer-containing pumps without dDAVP. Serum samples were collected from nine normonatremic (serum [Na+] = 147 ± 1.1 mmol/liter; plasma osmolality = 302 ± 1.5 mosmol/kg H2O) and 13 hyponatremic (serum [Na+] = 109.3 ± 1.1 mmol/liter; plasma osmolality = 236 ± 3 mosmol/kg H2O) rats after 3 months from the start of the experiment. Serum 8-hydroxy-2′-deoxyguanosine (8-OHdG) concentrations, a marker of oxidative DNA damage, were measured using a 96-well plate ELISA kit from Cell Biolabs, Inc. (San Diego, CA), according to the manufacturer's instructions on the Cell Biolabs Web site.
RESULTS
Osteoclastogenic Activity of Low Extracellular [Na+]
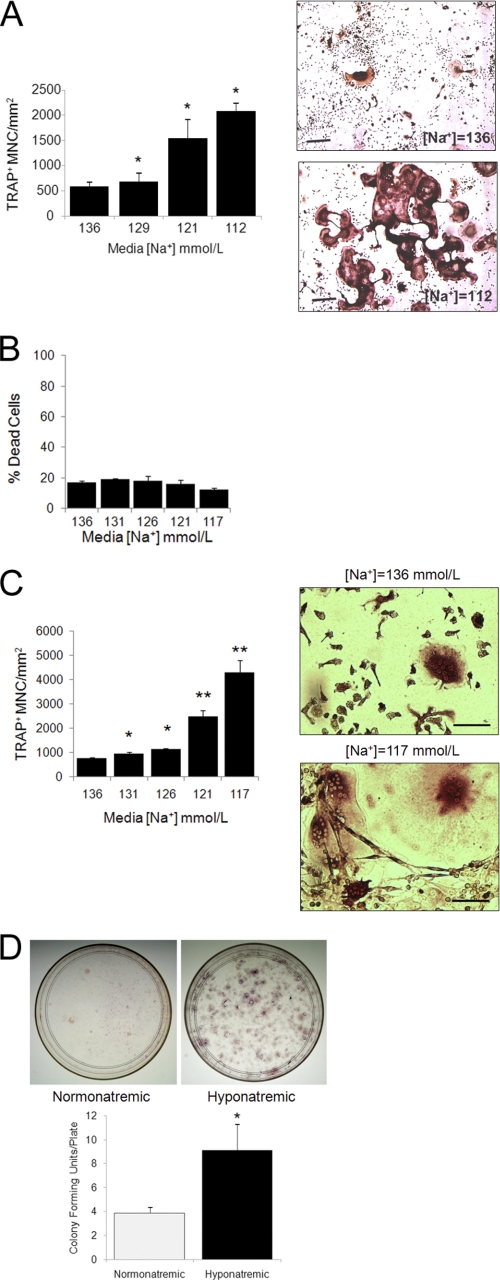
We explored the possibility that hyponatremia might have a direct effect on osteoclasts in two models: in a well established cellular model of murine RAW 264.7 preosteoclastic cells and in primary BMMs. RAW 264.7 cells form bone-resorbing osteoclasts when treated with recombinant RANKL and M-CSF (20–22). Using this model, we found that lowering the [Na+] in the culture media increased the number of mature osteoclasts (TRAP+ multinucleated cells) per mm2 of culture area. To mimic chronic hyponatremia, cells were fist adapted to grow in media with the desired [Na+] (graded concentrations between 136 and 112 mmol/liter) and were then cultured in differentiation media maintaining the same [Na+]. Results showed an inverse association between extracellular [Na+] and osteoclast number (Fig. 1A). Osteoclasts growing in low [Na+] were more numerous, larger, contained more nuclei, and were more interconnected than osteoclasts grown in normal [Na+] (see images in Fig. 1A). Additional time course studies compared osteoclastogenesis in medium with [Na+] = 112 mmol/liter and osteoclastogenesis in medium with normal [Na+]. After fixation, we counted the mature osteoclasts in triplicate wells on days 3, 5, 7, 11, and 14 and found that the osteoclast number increased in the samples with low extracellular [Na+] significantly after 3 days in culture (p < 0.05), and the difference was maximal after 11 days (p < 0.001) (data not shown). Culturing RAW 264.7 cells in reduced [Na+] media did not significantly alter cell viability as measured with the LIVE/DEAD assay. Fig. 1B shows [Na+]-dependent differences in dead cell number; the live cell assay did not show [Na+]-dependent changes either, except for a 15% increase of live cells at the lowest [Na+] (data not shown).
FIGURE 1.
Lowering [Na+] proportionally stimulates osteoclastogenesis. A, murine RAW 264.7 monocytic cells were grown in medium with the indicated [Na+] and treated for 7 days with recombinant RANKL (50 ng/ml) and M-CSF (10 ng/ml) to induce osteoclast differentiation. After fixation, cells were stained for TRAP activity, and quantification of TRAP-positive multinucleated cells was performed in triplicate wells. The graph (top) shows that lowering the [Na+] in the medium dose-dependently increased osteoclast formation. This direct effect was significant even with mild changes in [Na+] (e.g. 129 mmol/liter), a concentration that is associated with no symptoms in most patients. Data represent means ± S.E. (error bars). *, p < 0.01 comparing samples with one-step lower [Na+]. Representative images of TRAP-positive osteoclast cultures at medium [Na+] = 136 mmol/liter and medium [Na+] = 112 mmol/liter show marked differences of TRAP-positive cell density. Scale bars, 50 μm. B, RAW 264.7 cells were adapted to grow in media with the desired [Na+]. Equal numbers of cells were transferred to 96-well plates in triplicates. Cell viability was measured using a LIVE/DEAD cell kit. Calcein fluorescence emission intensities are expressed as percentage of control (emission from cells killed by incubation with digitonin according to the manufacturer's instructions). Data are expressed as mean ± S.E. No statistically significant differences were detected in viability of cells upon lowering [Na+]. C, BMMs were prepared from femora of four Sprague-Dawley rats, and monocytes were enriched by Ficoll gradient centrifugation, plated into 12-well culture dishes, adapted, and differentiated in media with target [Na+], as depicted on the graph. After 14 days, cells were fixed and stained for TRAP and counted. Shown are representative images from cells grown in medium with [Na+] = 136 mmol/liter and [Na+] = 117 mmol/liter; TRAP activity appears as a dark brown signal. Scale bars, 10 μm. Data are expressed as means ± S.E. from triplicates. *, p < 0.01; **, p < 0.001 comparing samples with one-step lower [Na+]. D, BMMs from hyponatremic and normonatremic rats were differentiated into osteoclasts while maintaining normal or low [Na+] as appropriate. Both the [Na+] = 117 mmol/liter and the [Na+] = 136 mmol/liter media were supplemented with M-CSF and RANKL. Images from representative 10-cm culture plates and the graph indicate that low [Na+] promoted formation of more TRAP+ colonies than normal [Na+]. Data are means ± S.E.; *, p < 0.001.
We found similar osteoclastogenic effects of reduced [Na+] culturing in experiments with primary BMMs (Fig. 1C). BMMs from normonatremic rats were adapted to grow in gradually lower [Na+] differentiation media. We found a [Na+]-dependent increase in mature osteoclast counts (Fig. 1C) and morphological changes similar to what we observed in the RAW 264.7 model, as depicted on representative images (Fig. 1C). Osteoclast counts were already increased significantly at moderately decreased [Na+] = 131 and [Na+] = 126 mmol/liter (p < 0.01), a range found in patients with mild, asymptomatic hyponatremia. To understand whether reduced [Na+] alters early or later stage osteoclastogenesis, we carried out an experiment on BMMs from seven rats kept hyponatremic (serum [Na+] = 110 ± 3 mmol/liter) and normonatremic (serum [Na+] = 142 ± 1.5 mmol/liter) for 2 weeks. The reduced [Na+] was maintained throughout BMM preparation and differentiation. We found a significant 3-fold increase of osteoclast counts on plates from hyponatremic BMMs compared with plates from normonatremic rats (data not shown). Interestingly, we found significantly more colonies of osteoclasts per 10-cm culture dish derived from hyponatremic than in dishes from normonatremic rats (Fig. 1D). BMMs are mixed colonies of osteoclastogenic cells at various stages of differentiation. The granulocyte-macrophage progenitors are multipotent cells that differentiate into osteoclasts with much higher efficiency than later stage, more committed macrophages. Therefore, the osteoclast colony-forming unit numbers indicate the proportion of early stage progenitors isolated from BMMs. Thus, these results are consistent with an effect of hyponatremia to increase formation of osteoclast progenitors.
Low Extracellular [Na+] Increases Osteoclastic Resorption
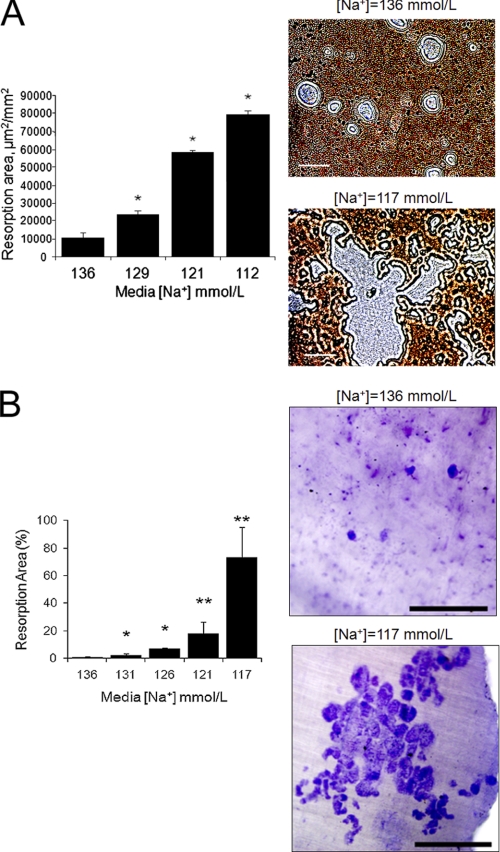
We found that reducing [Na+] increased resorbing activities proportionally to increased osteoclast formation from both RAW 264.7 cells and primary BMMs, using either OAAS plates or dentin slices as substrate. We first demonstrated that the increased number of RAW 264.7-derived osteoclasts formed in culture with a lower [Na+] functioned to degrade more calcium phosphate apatite in OAAS wells. Cells adapted to grow in media with the desired [Na+] were plated into OAAS wells and differentiated for 14 days. At the termination of the experiment, cells were removed, and the remaining calcium phosphate apatite coating was labeled with silver nitrate staining. Unstained resorption area per mm2 was determined from 25 random images taken from each well of triplicates, and results are shown as the mean resorption area/mm2 ± S.E. (Fig. 2A). The graph and representative images demonstrate that the unstained surface areas increased with lower medium [Na+] in a dose-dependent manner; the differences between the wells with low and normal [Na+] media were statistically significant (p < 0.01) even with small decreases of [Na+] to values that are analogous to serum [Na+] in patients with mild hyponatremia (129 mmol/liter). We then tested whether lowering extracellular [Na+] influences the resorbing activities of mature osteoclasts. The same number of RAW 264.7-derived mature osteoclasts, which were already differentiated for 7 days with either normal (142 mmol/liter) or low (115 mmol/liter) [Na+], were plated into triplicate calcium phosphate apatite-coated wells and cultured in normal or low [Na+] media without RANKL and M-CSF for an additional 7 days. Measurements of the resorptive areas showed a small but significant increase in the low [Na+] wells (4,909 ± 520 μm2/mm2) compared with the normal [Na+] wells (2,555 ± 430 μm2/mm2) (p < 0.01). In addition, we found that RAW 264.7 cells grown and differentiated in reduced [Na+] media ([Na+] = 131, 126, 121, and 117 mmol/liter) formed increasingly more resorption pits with larger resorption areas per total bone area (2.4-, 9.2-, 17.1-, and 47.7-fold, respectively) on dentin slices than cells grown in medium [Na+] = 136 mmol/liter (data not shown). Similarly, BMMs were differentiated in media with the same series of [Na+] for 14 days on dentin discs. Data representing mean ± S.E. of toluidine blue-positive (i.e. resorbed) areas demonstrated that reducing [Na+] dose-dependently increased resorption area (p < 0.01 for each, compared with [Na+] = 136 mmol/liter, control) (Fig. 2B). Representative images show resorption areas from discs with [Na+] = 136 and [Na+] = 117 mmol/liter, where the resorption areas are stained dark blue with toluidine blue. This increase in resorptive activity paralleled the increase in osteoclast number (compare with Fig. 1C).
FIGURE 2.
Lowering [Na+] stimulates resorptive activities of RAW 264.7 and bone marrow-derived osteoclastic cells. A, murine RAW 264.7 monocytic cells were grown in medium with the indicated [Na+] concentration in 24-well OAAS plates for 14 days with recombinant RANKL (50 ng/ml) and M-CSF (10 ng/ml). At the termination of the experiment, osteoclasts were removed, and the remaining calcium hydroxyapatite was stained using 5% silver nitrate under intense light exposure (dark). Resorbed surfaces (unstained areas) were assessed in triplicate wells, and the results show that the areas were inversely proportional to [Na+] in the extracellular medium. Data represent the means ± S.E. (error bars). *, p < 0.01 comparing samples with one-step higher [Na+]. Representative photomicrographs showing enlarged resorbed areas (gray) in wells where osteoclasts were cultured with normal or low [Na+] media. Scale bars, 50 μm. B, bone marrow macrophages were grown in differentiation media of decreasing [Na+], supplemented with M-CSF and RANKL for 5 days, and then overlaid onto whale dentin slices to differentiate another 9 days. Osteoclasts were removed, and dentin slices were stained with 1% toluidine blue to visualize resorption areas. The graph shows that resorption areas increased proportionally to lowered [Na+]. Average resorbed areas are expressed as a percentage of the total dentin area of two discs from each [Na+]. Data are mean ± S.D. *, p < 0.01; **, p < 0.001, compared with control samples ([Na+] = 136 mmol/liter). Representative images depicting [Na+]-dependent difference in resorbed areas (blue). Scale bars, 500 μm.
Osteoclastogenic Effects of Hyponatremia Involve Sodium Sensing Rather than Osmolality Sensing
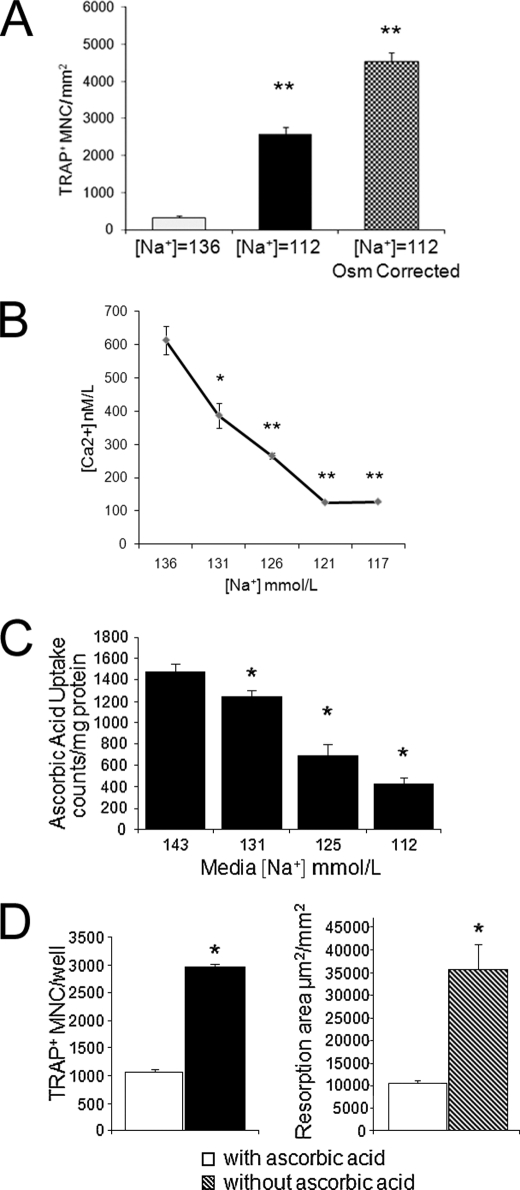
In the osteoclastogenesis and resorption experiments described above, the dilutional hyponatremia of SIADH with low extracellular sodium and hypo-osmolality was reproduced. To understand the underlying mechanisms by which osteoclastogenesis is triggered, we next manipulated the [Na+] in the culture media with and without correction of osmolality by the addition of mannitol. Results show that when we decreased both sodium and osmolality (from control [Na+] = 137 mmol/liter and osmolality = 290 mosmol/kg H2O to [Na+] = 112 mmol/liter and osmolality = 237 mosmol/kg H2O), this change increased the number of osteoclasts formed after 11 days per mm2 of culture area compared with control (p < 0.001). Surprisingly, growing RAW 264.7 cells in differentiation medium with low [Na+] (112 mmol/liter) while correcting osmolality to normal (290 mosmol/kg H2O) with the addition of mannitol did not decrease but rather increased the number of osteoclasts formed (p < 0.001) (Fig. 3A). Adjusting osmolality to normal also increased the resorptive activity of osteoclasts even more than low extracellular [Na+] without osmolality correction instead of preventing hyponatremia-induced osteoclast formation. Lowering both sodium and osmolality in the differentiation medium for 14 days increased the resorbed area by 372 ± 14% compared with control (p < 0.01), whereas lowering [Na+] and correcting osmolality to normal with the addition of mannitol increased the resorbed area by 966 ± 10% (p < 0.01). These findings indicated that changes in the activities of one or more extracellular [Na+]-dependent membrane channels would be likely candidates for signaling hyponatremia to osteoclasts. To interrogate this hypothesis, we searched the published literature for potential signaling pathways that could signal osteoclasts to respond to low extracellular [Na+], using the PubMed, GeneGo, and Ingenuity databases. Table 1 lists sodium-dependent channels that have been characterized in osteoclasts and how the normal activity of the given transporter influences osteoclastogenesis.
FIGURE 3.
Hyponatremia signaling associated with increased osteoclastogenesis. A, to test if osteoclastogenic effect of hyponatremia is related to sensing changes of osmotic pressure and cell swelling or to sensing changes in extracellular [Na+], RAW 264.7 cells were grown in medium with normal [Na+] (136 mmol/liter), in medium with both low [Na+] (112 mmol/liter) and uncorrected low osmolality (237 mosmol/kg H2O), or in medium with low [Na+] (112 mmol/liter) and corrected normal osmolality (290 mosmol/kg H2O) during differentiation. Cells were fixed and stained for TRAP activity, and TRAP+ multinucleated cells were counted. Data are mean ± S.E. (error bars) of triplicate samples. **, p < 0.001 compared with osteoclast counts in normonatremic samples. B, 2 × 106 RAW 264.7 cells adapted to grow in media with the indicated [Na+] were seeded into 96-well plates in triplicates and grown overnight. Cytosolic free [Ca2+] was measured using the Fluo-4 kit from Invitrogen, and calcium calibration was done using Fluo4 pentapotassium salt and standards from Invitrogen. Results show [Na+]-dependent decreases of cytosolic [Ca2+]. Data are mean ± S.E. (error bars). *, p < 0.01; **, p < 0.001 compared with [Ca2+] in control samples grown with medium [Na+] = 136 mmol/liter. C, RAW 264.7 cells grown in media with graded concentrations of [Na+] were subcultured into 24-well plates and then incubated with [14C]ascorbic acid for 30 min. After several washings with buffer containing unlabeled ascorbic acid, cells were solubilized, and radioactivity was counted. Values were corrected for protein content. Data represent means ± S.E. (error bars). *, p < 0.001. Experiments were in triplicate wells and repeated three times. The results show that lowering [Na+] in the media dose-dependently reduced ascorbic acid uptake into RAW 264.7 cells. D, RAW 264.7 cells were grown in minimal essential medium α without ascorbic acid or in the same medium supplemented with 50 mg/liter l-ascorbic acid and recombinant RANKL and M-CSF. TRAP-positive multinucleated cells were quantified after 7 days. Results show that the removal of ascorbic acid from the growth medium stimulates osteoclast formation. Similarly, cells were grown in OAAS resorption plates in media without and with ascorbic acid for 14 days, resorbed areas were quantified from triplicate wells, and the experiment was repeated three times. Data are means ± S.E. (error bars). *, p < 0.01. Results show that removal of ascorbic acid from the growth medium stimulates resorbing activity of RAW 264.7 osteoclastic cells.
TABLE 1.
Potential mediators of low [Na+] on osteoclasts
| Name | Transports | [Na+]-dependent effect on osteoclastogenesis |
|---|---|---|
| Sodium-dependent glutamate transporters | l-Glutamate, cysteine, aspartate | Stimulates early osteoclastogenesis (46) |
| Sodium-dependent ascorbic acid transporter | Ascorbic acid | Inhibits osteoclastogenesis (45) |
| Na+/Ca2+ exchanger | Calcium | Stimulates bone resorption (23) |
| Sodium-dependent phosphate co-transporters | Phosphate | Stimulates osteoclastogenesis (18) |
| Sodium bicarbonate co-transporter | Bicarbonate | Stimulates bone resorption (47) |
Lowering Extracellular [Na+] Decreases Cytosolic Free [Ca2+]
To test the hypothesis that [Na+]-dependent changes in the activity of the Na+/Ca2+ exchanger NCX mediate the signal of hyponatremia to osteoclasts, we measured intracellular [Ca2+] in two different experimental models. NCX is a bidirectional transporter that operates to influx Ca2+ and efflux Na+ in resorbing osteoclasts in forward mode or serves as a Na+ efflux mechanism in reverse mode. Previous reports indicated that removing extracellular [Na+] induced an intracellular [Ca2+] increase, and this effect, as well as the resorption pit area surrounding osteoclasts, could be suppressed by NCX inhibitors (23). Therefore, we expected that growing RAW 264.7 cells in reduced [Na+] would increase intracellular [Ca2+] via an NCX-mediated mechanism. In contrast, we found that cytosolic free [Ca2+] was proportionally lowered in a [Na+]-dependent manner (Fig. 3B). There was a small but significant (p < 0.01) decrease of cytosolic free [Ca2+] at moderately decreased [Na+] (131 and 126 mmol/liter) and a dramatic decrease at lower [Na+] (121 and 117 mmol/liter). These findings were from an experiment carried out in the presence of serum but were reproduced in another experiment using serum-free solutions, a cuvette system, and a monochromator-based fluorometer (data not shown). To test the hypothesis that depletion of endoplasmic reticulum calcium stores are responsible for the lowering of intracellular [Ca2+], we exposed cells to thapsigargin in the later experiment. Thapsigargin is a selective inhibitor of the endoplasmic reticulum calcium-dependent ATPase, known to induce a rapid and sustained rise in [Ca2+] by releasing [Ca2+] from the endoplasmic reticulum and inducing [Ca2+] influx (24). As expected, the addition of thapsigargin increased [Ca2+] in control cells grown with [Na+] = 136 mmol/liter. Interestingly, the addition of thapsigargin caused a larger rise of [Ca2+] in cells with lower [Na+]. The response increased by 29 ± 5% in cells with [Na+] = 126 mmol/liter but increased by 6.4-fold in cells with [Na+] = 121 and 8.17-fold in cells with [Na+] = 117 mmol/liter These findings indicate that [Na+]-dependent lowering of cytosolic free [Ca2+] is unlikely due to depletion of endoplasmic reticulum [Ca2+] stores.
Lowering Extracellular [Na+] Inhibits Ascorbic Acid Uptake into RAW 264.7 Cells
Another candidate for mediating the effects of hyponatremia on osteoclasts is the vitamin C transporter. The activity of active vitamin C transport has been shown to depend on the presence of extracellular [Na+] (25, 26). Ascorbic acid is a negative regulator of osteoclastogenesis and osteoclastic activity; thus, inhibition of this transporter could increase osteoclastogenesis and bone resorption. For vitamin C transporter to be a good candidate for sodium-sensing, ascorbic acid uptake would have to be sodium dose-dependent. Therefore, we tested the extracellular [Na+] dependence of the ascorbic acid uptake into RAW 264.7 cells using a well established assay to measure the uptake of 14C-labeled ascorbic acid. Our findings show that even small changes in [Na+] from 139 to 131 mmol/liter resulted in a significant (p < 0.05) decrease of radiolabeled ascorbic acid uptake, and gradual lowering of [Na+] resulted in an even greater proportional decrease of ascorbic acid uptake (Fig. 3C).
These results support the possibility that sodium-dependent ascorbic acid uptake may be one of the mechanisms by which osteoclastogenesis and osteoclastic activity is inversely regulated by extracellular sodium concentration. We tested this possibility by measuring the number of osteoclasts formed and the area of calcium-phosphate apatite surface resorbed when RAW 264.7 cells were differentiated in media depleted of or supplemented with ascorbic acid. Removal of ascorbic acid from the culture medium mimicked the enhanced osteoclastogenic and bone-resorbing effects of low [Na+] (p < 0.001 for both assay results) (Fig. 3D).
Low Extracellular [Na+] Induces Accumulation of Free Oxygen Radicals and Elicits Oxidative Stress Responses
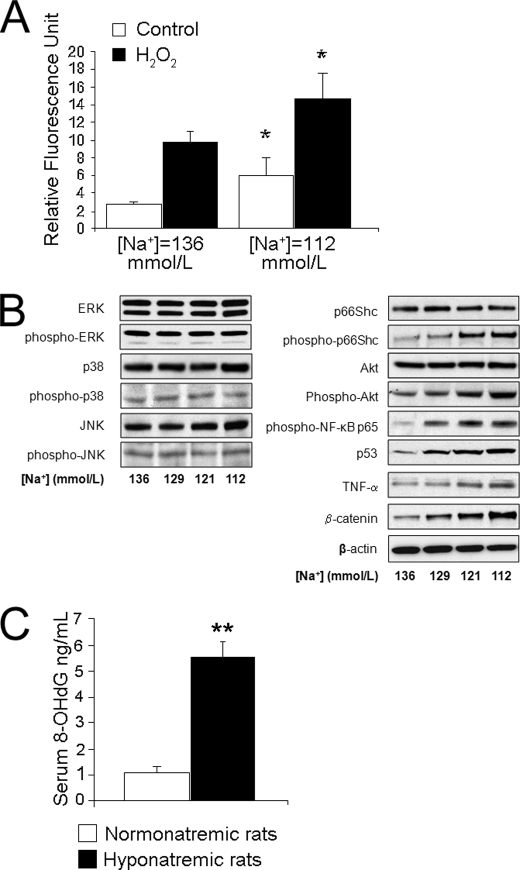
Intracellular ascorbic acid is a free radical quencher, part of the defense mechanisms to protect cells, including osteoclastic cells. Hence, we tested the hypothesis that low [Na+]-induced deficiency of ascorbic acid uptake results in increased accumulation of free radicals in osteoclasts. We measured free radical formation over 30 min in cells cultured in media with normal or low [Na+] by a well established fluorescence assay that monitors the oxidation of DCF. Results indicated that, compared with free radical formation in control cells, cells cultured in medium with [Na+] = 112 mmol/liter generated 3-fold higher amounts of fluorescing DCF in 30 min (p < 0.001) (Fig. 4A). We then measured the free radical generation during an oxidative challenge by adding hydrogen peroxide (H2O2) to the culture media (Fig. 4A). As expected, treatment with H2O2 doubled the accumulation of DCF fluorescence in cells cultured in medium with [Na+] = 136 mmol/liter (normal) and, even more, to 7-fold stimulation in cells cultured with medium with [Na+] = 112 mmol/liter (p < 0.01 comparing normal and low [Na+] with H2O2). These results indicate that lowering extracellular [Na+] induces free oxygen radical accumulation and weakens the cellular protection against O2 free radicals.
FIGURE 4.
Lowering [Na+] increases accumulation of reactive oxygen species and elicits oxidative stress response in cellular and animal models of hyponatremia. A, RAW 264.7 cells were grown in media with the indicated [Na+] and normalized osmolality; cells were subcultured into 96-well plates and the next day were incubated with DCF with and without H2O2 for 30 min and the Hoescht 33342 dye for the last 5 min. DCF fluorescence was measured using a fluorometer plate reader (Tecan US Inc., model Ultra384) and corrected with values of Hoechst fluorescence (DNA content). Data represent means ± S.E. (error bars). *, p < 0.01. Results show that lowering the [Na+] concentration in the media increased base-line and peroxide-induced accumulation of reactive oxygen species, an indicator of oxidative stress. B, whole cell lysates were generated from RAW 264.7 cells grown for 48 h in media with the specified [Na+] without the correction of osmolality and probed for the abundance of key signaling molecules in multiple osteoclastogenesis pathways as described under “Experimental Procedures.” β-Actin was used to confirm equal protein loading. Blots on the left indicate no change in three of five key RANKL-induced pathways, whereas blots on the right indicate that lowering extracellular [Na+] proportionally increases the phosphorylation of key proteins in the Akt and NF-κB pathways common to both RANKL and ROS signaling. The abundance of additional key molecules belonging to the oxidative stress signaling cascade was proportionally increased by lowering [Na+]. C, sera from hyponatremic and normonatremic rats were collected as described under “Experimental Procedures.” 8-OHdG concentrations were determined in duplicates using an ELISA kit. Data represent means ± S.E. (error bars). **, p < 0.01.
Hyponatremia-induced oxidative stress was manifested by changes of protein phosphorylation and expression, as indicated by Western blot analysis. These changes were proportional to the degree of lowering of the sodium concentrations in the culture media. ROS is one of the most important modulators of RANKL-induced osteoclastogenesis. ROS accumulation has been reported to activate several signaling pathways common to RANKL signaling and to up-regulate stress, survival, and apoptosis-related genes (27). RANKL binding to RANK activates five distinct signaling cascades mediated by MAPKs, including JNK, p38 MAP kinase, ERK, NF-κB, and Akt survival pathways (28–31). Using Western blot analyses of extracts from RAW 264.7 cells, we found that lowering [Na+] did not alter the expression or phosphorylation of the first three kinases (Fig. 4B, left). Another two pathways of RANKL signaling were altered by lowering [Na+], the Akt survival kinase and the NF-κB (Fig. 4B, right). We found evidence supporting activation of additional ROS-dependent pathways, an inverse relationship between extracellular medium [Na+] (136, 129, 121, and 112 mmol/liter) and the phosphorylation of the longevity-associated p66Shc (32) and the expression of p53 (33), β-catenin (34), and TNF-α (35) (Fig. 4B). These findings all support the hypothesis that oxidative stress responses are triggered by lowering the extracellular [Na+].
Finally, we tested the in vivo relevance of this oxidative stress response to low extracellular [Na+] in our rat model of chronic hyponatremia by measuring a marker of oxidative DNA damage, 8-OHdG, in the sera of 22-month-old female rats as described under “Experimental Procedures.” Results indicate that the concentration of 8-OHdG in the sera from hyponatremic rats was more than 5-fold higher than in the sera from normonatremic rats (Fig. 4C). This finding is consistent with the hypothesis that a low [Na+] triggers osteoclastic bone resorption by signaling through the oxidative stress pathway, not only in cultured RAW 264.7 cells but also in our rat model of SIADH.
DISCUSSION
Our experiments demonstrate for the first time that chronic lowering of extracellular [Na+] directly stimulates osteoclastogenesis and osteoclastic bone resorption. This response is necessary to liberate stored sodium from the bone matrix in an attempt to restore normal extracellular [Na+]. The direct effect of extracellular [Na+] on osteoclastogenesis and osteoclastic resorption very likely to contribute to the osteoporosis associated with hyponatremia in our animal model of chronic hyponatremia (3), and similarly to the increased risk of osteoporosis and fractures in humans with chronic hyponatremia (1–3).
Marked concentration-dependent osteoclastogenic effects of hyponatremia were demonstrated by studies in two different systems: in RAW 264.7 cell-derived and primary BMM cultures. Remarkably, BMM differentiation studies from normonatremic and hyponatremic rats indicated that low [Na+] stimulates differentiation of early stage osteoclast progenitors, indicated by markedly increased colony formation from BMMs of hyponatremic rats (Fig. 1D). This effect may be related to [Na+]-dependent increases in M-CSF sensitivity due to oxidative stress (Fig. 4), as suggested for age-related increases of osteoclast progenitors (36, 37).
Results of our cell culture studies indicate that cellular responses to low extracellular [Na+], rather than to low extracellular osmolality, represent the dominant mechanism underlying hyponatremia-induced osteoclastogenesis and bone resorptive activity. In fact, hypoosmolality actually somewhat mitigated the osteoclastogenic effect of hyponatremia, probably through cell swelling-induced activation of the outwardly rectifying chloride ion channel (38, 39). Differentiation of osteoclasts involves fusion of bone marrow macrophage mononuclear precursors in response to extracellular signals. A dramatic increase in osteoclast cell volume occurs during this osteoclast biogenesis. This effect in conjunction with hypo-osmolality-induced cell swelling activates stretch- and swelling-activated cation channels (40), thus increasing Ca2+ influx and intracellular calcium (41), which in turn promotes osteoclast apoptosis (24) and inhibits osteoclastic bone resorption (42–44). Diverse agents, such as calcitonin, IL4, and protein-tyrosine kinase inhibitors, have been shown to inhibit bone resorption of mammalian osteoclasts by increasing intracellular [Ca2+]. Our finding that adaptation to low [Na+] decreased intracellular [Ca2+] in RAW 264.7 cells is also consistent with a [Na+]-sensing rather than an osmolality-sensing mechanism of the osteoclastogenic effect of hyponatremia.
Both the osteoclastogenic and resorptive responses to low [Na+] were proportional to the concentration of sodium in the culture media. This strongly suggested that osteoclasts have a sensory mechanism or receptor that can transmit the signal of extracellular [Na+]. Such mechanism has not yet been described in cell types of the osteoclastogenic lineage or in any other cell types. The possibility of sodium sensors in the central nervous system as well as in the kidney have been explored previously, but this quest was largely abandoned in the 1980s because it was conceptually difficult to imagine that a receptor could respond to small changes in ionic concentrations in the millimolar range. The concept that cells and organisms have developed sensors to ions has again surfaced following the successful cloning of the calcium-sensing receptor in the 1990s but has not yet yielded discovery of a sodium-sensing receptor. On the other hand, the existence of sodium-gated ion channels and transporters, which could serve as surrogate [Na+] sensors, is well established (see Table 1).
Of these five transporters known to be expressed and function in osteoclasts, both the Na+/Ca2+ exchanger NCX (23) and the ascorbic acid transporter (45) are likely candidates for possible [Na+] sensors. The others, including the glutamate (46), the phosphate (18), and the bicarbonate (47) transporters, are less likely candidates because their inhibition by lowering extracellular [Na+] would attenuate rather than stimulate osteoclastic resorption. Our findings on intracellular [Ca2+] lowering by long term removal of extracellular [Na+] make it unlikely that NCX mediates intracellular Ca2+ increase. Previous reports indicated that acute removal of [Na+] increased [Ca2+] influx and intracellular [Ca2+] and that application of NCX inhibitors or siRNA targeting of NCX inhibited bone resorption in mouse osteoclasts (23). An interesting possibility to explore is that chronic lowering of extracellular [Na+] would lead to up-regulation of NCX expression and increased forward transport to extrude [Ca2+].
Here we show for the first time that the activity of the sodium-dependent ascorbic acid transporter is modulated by graded concentrations of extracellular [Na+]. Inhibition of ascorbic acid transporter by removal of extracellular [Na+] and the effect of ascorbic acid to protect from ROS accumulation and oxidative stress are well documented in osteoblasts (48) and in osteoclasts (45). As expected, lowering sodium in the extracellular media not only reduced ascorbic acid uptake but also increased reactive oxygen radical accumulation in osteoclastic cells and triggered changes in protein expression consistent with an oxidative stress response. Moreover, we found increased concentrations of oxidative DNA damage product 8-OHdG in the sera of hyponatremic rats, compared with normonatremic rats, indicating that oxidative stress response is probably a relevant mechanism for chronic hyponatremia-induced pathological changes in vivo. Importantly, markers of oxidative stress have been found to be inversely associated with bone mineral density in human studies (49).
Oxidative stress is also a widely accepted mechanistic explanation for aging. Most of the reactive oxygen species (superoxide, hydrogen peroxide, and hydroxyl radicals) are generated in the mitochondria as a by-product of glucose metabolism and ATP generation. Hydrogen peroxide increases the permeability of the mitochondrial membrane and causes cell swelling and apoptosis. Defense mechanisms against oxidative stress involve enzymes (superoxide dismutase, glutathione peroxidase, reductases, catalases), antioxidants, and changes in the expression of several cyclins, cyclin-dependent kinase inhibitors, DNA repair, and apoptosis control genes as well as antioxidant enzymes. Proteins that defend against oxidative stress, including forkhead box transcription factors (50) and β-catenin (34), are indispensable for maintaining normal bone mass (51). Recent research has demonstrated that aging weakens these defenses, leading to progressive osteoporosis (51). Oxidative stress has already been linked with several forms of osteoporosis, not just aging-related osteoporosis. Excessive production of free radicals and reactive oxygen species stimulates osteoclastogenesis and osteoclastic bone resorption by triggering oxidative stress and is a critical mechanism involved with increases in bone resorption induced by estrogen deficiency, androgen deficiency, and inflammatory cytokines. Because chronic hyponatremia is most prevalent in the aging population, who are also most affected by hypogonadism and chronic inflammatory conditions, it seems possible that these combined comorbidities lead to critical challenges to the defense mechanisms against oxidative stress, thereby accelerating the aging process.
In summary, our findings represent important initial insights into the molecular mechanisms by which chronic hyponatremia promotes bone loss. Based on our histomorphometric data from hyponatremic rats and findings that hyponatremia directly activates osteoclasts in this cellular model, antiresorptive treatment in patients with hyponatremia and low bone mass should be considered for prospective randomized clinical trials. Until recently, no effective and tolerable treatment was available for mild hyponatremia. The recent approval of antagonists of the vasopressin V2 receptor offers the likelihood that hyponatremia will be correctable in the near future (52). Therefore, understanding the mechanisms involved in the long term adverse effects of hyponatremia is both timely and important. Future studies will probably reveal additional signaling mechanisms activated by low [Na+]. The immediate next step will be to explore the differential gene expression profile of osteoclasts exposed to low [Na+]. Our preliminary results using this approach indicate that increased sensitivity to calcitriol and down-regulation of glutathione biosynthesis could be major contributors. Other studies expected to lead to a better understanding into sodium sensing involve identifying the putative sodium-sensing receptor, which is likely to be expressed in multiple tissues. Such a receptor should be well conserved between plants and various eukaryotic cells because adaptation to changes in salinity of the soil and water is a general requirement of adaptation to maintain homeostasis in response to varying environmental conditions.
Acknowledgments
We are grateful to David G. Roodman for the kind gift of whale bone slices. We thank Lisa Linde, Thurston Sandberg, Aifen Wang, Deborah L. Berry, and Nicole Jackson for expert technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant Z01-DK060050 and Extramural Grant R01-AG029477. This work was also supported by a research grant from Yamanouchi Pharma America (Paramus, NJ).
- SIADH
- syndrome of inappropriate antidiuretic hormone secretion
- RANK
- receptor activator of NF-κB
- RANKL
- RANK ligand
- M-CSF
- macrophage colony-stimulating factor
- TRAP
- tartrate-resistant acid phosphatase
- BMM
- bone marrow monocyte
- AVP
- arginine vasopressin
- DCF
- 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate
- 8-OHdG
- 8-hydroxy-2′-deoxyguanosine
- dDAVP
- desmopressin
- ROS
- reactive oxygen species.
REFERENCES
- 1. Gankam Kengne F., Andres C., Sattar L., Melot C., Decaux G. (2008) QJM 101, 583–588 [DOI] [PubMed] [Google Scholar]
- 2. Sandhu H. S., Gilles E., DeVita M. V., Panagopoulos G., Michelis M. F. (2009) Int. Urol. Nephrol. 41, 733–737 [DOI] [PubMed] [Google Scholar]
- 3. Verbalis J. G., Barsony J., Sugimura Y., Tian Y., Adams D. J., Carter E. A., Resnick H. E. (2010) J. Bone Miner. Res. 25, 554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergstrom W. H., Wallace W. M. (1954) J. Clin. Invest. 33, 867–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seeliger E., Ladwig M., Reinhardt H. W. (2006) Am. J. Physiol. Regul. Integr. Comp Physiol. 290, R1429–R1435 [DOI] [PubMed] [Google Scholar]
- 6. Blair H. C., Zaidi M. (2006) Rev. Endocr. Metab Disord. 7, 23–32 [DOI] [PubMed] [Google Scholar]
- 7. Roodman G. D., Windle J. J. (2005) J. Clin. Invest. 115, 200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu Y., Morse L. R., da Silva R. A., Odgren P. R., Sasaki H., Stashenko P., Battaglino R. A. (2010) Antioxid. Redox Signal. 13, 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharif-Naeini R., Ciura S., Zhang Z., Bourque C. W. (2008) Kidney Int. 73, 811–815 [DOI] [PubMed] [Google Scholar]
- 10. Verbalis J. G. (2007) J. Am. Soc. Nephrol. 18, 3056–3059 [DOI] [PubMed] [Google Scholar]
- 11. Tian W., Fu Y., Garcia-Elias A., Fernández-Fernández J. M., Vicente R., Kramer P. L., Klein R. F., Hitzemann R., Orwoll E. S., Wilmot B., McWeeney S., Valverde M. A., Cohen D. M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14034–14039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sjöström M., Stenström K., Eneling K., Zwiller J., Katz A. I., Takemori H., Bertorello A. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 16922–16927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bize V., Horisberger J. D. (2007) Am. J. Physiol. Renal Physiol. 293, F1137–F1146 [DOI] [PubMed] [Google Scholar]
- 14. Perry S. F., Rivero-Lopez L., McNeill B., Wilson J. (2006) J. Exp. Biol. 209, 4591–4596 [DOI] [PubMed] [Google Scholar]
- 15. Almeida M., Han L., Martin-Millan M., Plotkin L. I., Stewart S. A., Roberson P. K., Kousteni S., O'Brien C. A., Bellido T., Parfitt A. M., Weinstein R. S., Jilka R. L., Manolagas S. C. (2007) J. Biol. Chem. 282, 27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Srivastava S., Toraldo G., Weitzmann M. N., Cenci S., Ross F. P., Pacifici R. (2001) J. Biol. Chem. 276, 8836–8840 [DOI] [PubMed] [Google Scholar]
- 17. Davey R. A., Turner A. G., McManus J. F., Chiu W. S., Tjahyono F., Moore A. J., Atkins G. J., Anderson P. H., Ma C., Glatt V., MacLean H. E., Vincent C., Bouxsein M., Morris H. A., Findlay D. M., Zajac J. D. (2008) J. Bone Miner. Res. 23, 1182–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gupta A., Guo X. L., Alvarez U. M., Hruska K. A. (1997) J. Clin. Invest. 100, 538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shioi A., Ross F. P., Teitelbaum S. L. (1994) Calcif. Tissue Int. 55, 387–394 [DOI] [PubMed] [Google Scholar]
- 20. Huber D. M., Bendixen A. C., Pathrose P., Srivastava S., Dienger K. M., Shevde N. K., Pike J. W. (2001) Endocrinology 142, 3800–3808 [DOI] [PubMed] [Google Scholar]
- 21. Shui C., Riggs B. L., Khosla S. (2002) Calcif. Tissue Int. 71, 437–446 [DOI] [PubMed] [Google Scholar]
- 22. Wei S., Teitelbaum S. L., Wang M. W., Ross F. P. (2001) Endocrinology 142, 1290–1295 [DOI] [PubMed] [Google Scholar]
- 23. Li J. P., Kajiya H., Okamoto F., Nakao A., Iwamoto T., Okabe K. (2007) Endocrinology 148, 2116–2125 [DOI] [PubMed] [Google Scholar]
- 24. Yip K. H., Zheng M. H., Steer J. H., Giardina T. M., Han R., Lo S. Z., Bakker A. J., Cassady A. I., Joyce D. A., Xu J. (2005) J. Bone Miner. Res. 20, 1462–1471 [DOI] [PubMed] [Google Scholar]
- 25. Sotiriou S., Gispert S., Cheng J., Wang Y., Chen A., Hoogstraten-Miller S., Miller G. F., Kwon O., Levine M., Guttentag S. H., Nussbaum R. L. (2002) Nat. Med. 8, 514–517 [DOI] [PubMed] [Google Scholar]
- 26. Franceschi R. T., Wilson J. X., Dixon S. J. (1995) Am. J. Physiol. 268, C1430–C1439 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y., Fong C. C., Wong M. S., Tzang C. H., Lai W. P., Fong W. F., Sui S. F., Yang M. (2005) Apoptosis. 10, 545–556 [DOI] [PubMed] [Google Scholar]
- 28. Schreck R., Rieber P., Baeuerle P. A. (1991) EMBO J. 10, 2247–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim H. J., Chang E. J., Kim H. M., Lee S. B., Kim H. D., Su Kim G., Kim H. H. (2006) Free Radic. Biol. Med. 40, 1483–149316632109 [Google Scholar]
- 30. Niwa K., Inanami O., Yamamori T., Ohta T., Hamasu T., Kuwabara M. (2003) Antioxid. Redox Signal. 5, 713–722 [DOI] [PubMed] [Google Scholar]
- 31. Roodman G. D. (2006) Ann. N.Y. Acad. Sci. 1068, 100–109 [DOI] [PubMed] [Google Scholar]
- 32. Migliaccio E., Giorgio M., Mele S., Pelicci G., Reboldi P., Pandolfi P. P., Lanfrancone L., Pelicci P. G. (1999) Nature 402, 309–313 [DOI] [PubMed] [Google Scholar]
- 33. Sablina A. A., Budanov A. V., Ilyinskaya G. V., Agapova L. S., Kravchenko J. E., Chumakov P. M. (2005) Nat. Med. 11, 1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Essers M. A., de Vries-Smits L. M., Barker N., Polderman P. E., Burgering B. M., Korswagen H. C. (2005) Science 308, 1181–1184 [DOI] [PubMed] [Google Scholar]
- 35. Lee N. K., Choi Y. G., Baik J. Y., Han S. Y., Jeong D. W., Bae Y. S., Kim N., Lee S. Y. (2005) Blood 106, 852–859 [DOI] [PubMed] [Google Scholar]
- 36. Baek K. H., Oh K. W., Lee W. Y., Lee S. S., Kim M. K., Kwon H. S., Rhee E. J., Han J. H., Song K. H., Cha B. Y., Lee K. W., Kang M. I. (2010) Calcif. Tissue Int. 87, 226–235 [DOI] [PubMed] [Google Scholar]
- 37. Perkins S. L., Gibbons R., Kling S., Kahn A. J. (1994) Bone 15, 65–72 [DOI] [PubMed] [Google Scholar]
- 38. Sakuta K., Morihata H., Mori H., Sakai H., Kuno M. (2002) Osaka City Med. J. 48, 29–38 [PubMed] [Google Scholar]
- 39. Kelly M. E., Dixon S. J., Sims S. M. (1994) J. Physiol. 475, 377–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Komarova S. V., Dixon S. J., Sims S. M. (2001) Curr. Pharm. Des. 7, 637–654 [DOI] [PubMed] [Google Scholar]
- 41. Christensen O. (1987) Nature 330, 66–68 [DOI] [PubMed] [Google Scholar]
- 42. Tsuzuki T., Okabe K., Kajiya H., Habu T. (2000) Jpn. J. Physiol. 50, 67–76 [DOI] [PubMed] [Google Scholar]
- 43. Datta H. K., MacIntyre I., Zaidi M. (1990) Biochem. Biophys. Res. Commun. 167, 183–188 [DOI] [PubMed] [Google Scholar]
- 44. Kim M. S., Yang Y. M., Son A., Tian Y. S., Lee S. I., Kang S. W., Muallem S., Shin D. M. (2010) J. Biol. Chem. 285, 6913–6921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xiao X. H., Liao E. Y., Zhou H. D., Dai R. C., Yuan L. Q., Wu X. P. (2005) J. Endocrinol. Invest. 28, 253–260 [DOI] [PubMed] [Google Scholar]
- 46. Skerry T. M. (2008) J. Musculoskelet. Neuronal. Interact. 8, 166–173 [PubMed] [Google Scholar]
- 47. Riihonen R., Nielsen S., Väänänen H. K., Laitala-Leinonen T., Kwon T. H. (2010) Matrix Biol. 29, 287–294 [DOI] [PubMed] [Google Scholar]
- 48. Wilson J. X., Dixon S. J. (1989) J. Membr. Biol. 111, 83–91 [DOI] [PubMed] [Google Scholar]
- 49. Basu S., Michaëlsson K., Olofsson H., Johansson S., Melhus H. (2001) Biochem. Biophys. Res. Commun. 288, 275–279 [DOI] [PubMed] [Google Scholar]
- 50. Nemoto S., Finkel T. (2002) Science 295, 2450–2452 [DOI] [PubMed] [Google Scholar]
- 51. Manolagas S. C. (2010) Endocr. Rev. 31, 266–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schrier R. W., Gross P., Gheorghiade M., Berl T., Verbalis J. G., Czerwiec F. S., Orlandi C. (2006) N. Engl. J. Med. 355, 2099–2112 [DOI] [PubMed] [Google Scholar]