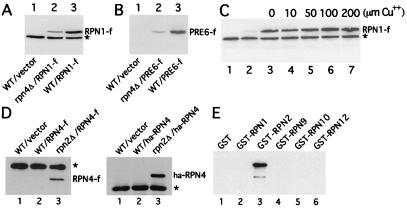
Figure 1.
RPN4 is required for normal expression of proteasomal subunits. (A and B) Immunoblot analysis of RPN1 (A) and PRE6 (B) that were C-terminally tagged with flag epitope and expressed from their native promoters and a low-copy plasmid in a rpn4Δ S. cerevisiae strain (lane 2) and its congenic wild-type counterpart JD52 (lane 3). Lane 1 in A and B, cells transformed with empty vector. (C) Enhanced expression of RPN4 marginally elevates the level of RPN1. rpn4Δ cells were cotransformed with low-copy plasmids expressing, respectively, RPN1-flag and RPN4 from the PCUP1 promoter. Increasing concentrations of CuSO4 were used to induce the expression of RPN4 (lanes 3–7). Lanes 1 and 2 in C, rpn4Δ cells transformed, respectively, with empty RPN1-flag vector and empty RPN4 vector. (D) Immunoblot analyses of C-terminally (RPN4-flag) or N-terminally (ha-RPN4) tagged RPN4 that was expressed from the induced PCUP1 promoter and low-copy plasmid either in wild-type (RPN4) strain JD52 or in AVY302, a congenic rpn2Δ mutant (see legend to Fig. 2 for details). (E) RPN4 interacts with RPN2 in GST-pulldown assays. Extracts of E. coli expressing RPN4-flag were incubated with glutathione-agarose beads preloaded with the indicated GST fusions or GST alone. The retained proteins were eluted, fractionated, and immunoblotted with anti-FLAG antibody. Approximately equal amounts of different GST fusions were immobilized on glutathione-agarose beads in these assays, as verified by Coomassie staining (data not shown). SDS/PAGE in 6, 8, and 12% gels was used, respectively, in A and C, in D and E, and in B. The asterisk in A, C, and D indicates a crossreacting band.