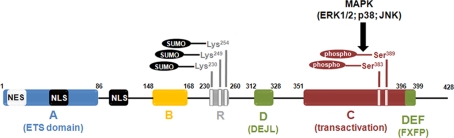
Figure 1.
Diagram illustrating functional domains and major post-translational modifications of the Elk-1 protein. The ETS domain (or A) domain lies within the n-terminus end of the protein and is responsible for Elk-1 binding to DNA. The B domain is involved in the binding of Elk-1 to a dimer of its cofactor, the SRF. The R domain is crucial for the repression of Elk-1 transcriptional activity. It contains the lysine residues that are susceptible to be SUMOylated, a post-translational event that reinforce the repression exerted by the R domain. The C (or transactivation) domain contains the amino acids that are phosphorylated by MAP kinases. Among these residues, phosphorylation of Serine 383 and Serine 389 is a crucial event to activate Elk-1-mediated transcription. The two domains depicted in green are involved in the binding of Elk-1 to activated MAP kinases. The D (or DEJL) domain is responsible for the binding of Elk-1 to activated MAP kinases of the ERK, JNK, and p38 subtypes. The DEF (or FXFP) domain is more specific since it is only required for the binding of Elk-1 to activated ERK. The NES and NLS motifs are involved in nuclear export and import of Elk-1, respectively.