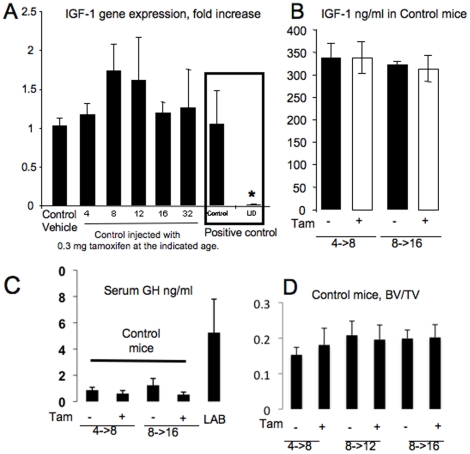
Figure 1. Validation of the iLID model.
(A) Liver expression of IGF-1 in control mice (mean ± SE) is not altered following 0.3 mg tamoxifen injection. Control mice at 4, 8, 12, 16 and 32 weeks of age were injected with 0.3 mg tamoxifen and livers were dissected four weeks later for gene expression analysis using real time PCR (n = 3 per group). Control and “constitutive” LID mice were used as positive control for the assay. Cycle number was corrected to β-actin and levels related to vehicle control, which was set as 1. (B) Mean serum IGF-1 levels in control mice (± SE) injected with 0.3 mg of tamoxifen (+) at 4 or 8 weeks were not altered compared to vehicle treated controls (−) at 8 or 16 weeks, respectively. (C) Mean serum GH levels in control mice (± SE) injected with 0.3 mg of tamoxifen (+) at 4 or 8 weeks were not altered compared to vehicle treated controls (−) at 8 or 16 weeks, respectively. Sera from triple knockout of LID/acid labile subunit KO/IGFBP-3KO (LAB) mice, which have high serum GH levels [24], served as a positive control. (D) BV/TV (bone volume/total volume) from micro-CT analysis of control mice injected with vehicle (−) or 0.3 mg tamoxifen (+) at 4, 8, and 12 weeks of age and then analyzed at 8, 12, and 16 weeks, respectively. Trabecular bone was analyzed at the femoral distal metaphysis (n = 6 per group). No significant differences were found between vehicle and 0.3 mg tamoxifen injected mice.