1. Introduction
Glaucoma is a disease that results in degeneration of the optic nerve, and it is a leading cause of blindness in humans and animals (Quigley, 1996). The most important risk factor for glaucoma is elevated intraocular pressure (IOP) (Lesky, 1983), and current treatment strategies for glaucoma target lowering and regulating IOP (AGIS Investigators, 2000). The ability to accurately measure IOP is of central importance to the diagnosis, monitoring of therapeutic response and progression of glaucoma, in both clinical and research settings. Therefore, it is essential to have accurate techniques available to measure IOP in non-human primates, as they are commonly used as an animal model in glaucoma research (Rasmussen and Kaufman, 2005; Weinreb and Lindsey, 2005).
In recent years, a hand-held tonometer based on the induction/impact principle first described by Kontiola has become commercially available (Kontiola, 1996-1997, 2000). The TonoVet® uses rebound tonometry to measure IOP (Wang et. al., 2005). This handheld, portable instrument uses an electromagnetically propelled metal probe with a plastic tip to contact the corneal surface. As the metal probe rebounds back into the device, the velocity of the probe is sensed and used to estimate IOP. Because rebound tonometry makes very brief contact with the corneal surface, the need for topical anesthesia is eliminated. Rebound tonometry has been found to provide reliable and accurate IOP measurements in humans (Abraham et.al., 2008; Filippopoulos et. al., 2006), dogs (Görig et. al., 2006; Knollinger et. al., 2005; Leiva et. al., 2006); rabbits (Kalesnykas and Uusitalo, 2007); mice (Kim et. al., 2007); birds of prey (Jeong et. al., 2007; Reuter et. al., 2010); and rhesus monkeys (Yu et. al., 2009). At the time our study was performed results from testing in any non-human primates had yet to be published. Since cynomolgus macaques play a vital role in translational research, representing important models for research into human ocular diseases, including glaucoma (Gonnering et. al., 1983; Lee et. al., 1987; Serle et. al., 1998), evaluation of rebound tonometry in this species is essential. In the current study, we compared readings taken with the TonoVet® (Tiolat Oy, Helsinki, Finland) rebound tonometer to manometrically set IOP and to readings taken by ‘minified’ Goldmann applanation tonometry (Kaufman and Davis, 1980) in cynomolgus macaques. We also evaluated the effects of central corneal thickness on measurements obtained by the TonoVet®.
2. Methods
2.1 Animals and Anesthesia
Two male and 1 female cynomolgus macaque (Macaca fascicularis), aged 9 – 10 years, and weighing between 5.0 and 9.7kg were used in this study. Animals were determined to be free of ocular abnormalities by slit-lamp biomicroscopy prior to any procedure. Animals were anesthetized with intramuscular ketamine HCl (10-15mg/kg), followed by intravenous pentobarbital sodium (10-15 mg/kg initial, 5-10mg/kg supplemental). All animal experiments were performed with approval of the institutional animal care and use committee, and in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2 Intraocular Pressure Manipulations and Measurements
Baseline IOP readings were obtained with the ‘minified’ Goldmann applanation tonometer (GAT) and the TonoVet®. The anterior chamber of each eye was cannulated with a branched 23-gauge needle. One arm of each needle was attached via polyethylene tubing to a vertically adjustable open reservoir filled with Bárány’s mock aqueous humor (Bárány, 1964); the other arm of the needle was attached via polyethylene tubing to a pressure transducer calibrated as previously described (Bárány and Rohen, 1965). The pressure transducer was connected to an amplifier that was attached to a computer monitoring voltages and to a chart recorder. Manometric IOP values taken from the chart recorder tracing were used in data analysis. Voltages from the computer verified the IOP values on the tracing.
Intraocular pressure was increased in both eyes by 5mmHg increments from 5mmHg to 70 mmHg, and then lowered by 10mmHg decrements, from 70mmHg to 10mmHg, by raising and lowering the reservoir, respectively. Two separate unmasked operators each obtained three consecutive IOP readings in both eyes with the TonoVet® at each 5mmHg increment and at each 10mmHg decrement. Though our experimenters were ‘unmasked’, the TonoVet® was used according to the manufacturer’s instructions, minimizing the potential for operator-introduced bias. Only the first 3 readings considered “acceptable” by the TonoVet® (that is, within the range of acceptable standard deviation as indicated by no bars or one bar on the instrument display) were reported. The right eye was measured first and operator order remained consistent. A single IOP reading was obtained for each eye by a single, consistent operator (one of the two TonoVet® operators above) using the GAT, at alternating increments, beginning at 5mmHg and at every 10mmHg decrement. Central corneal thickness was measured using a PachPen™ ultrasonic pachymeter (Oculab, Glendale, CA, USA) by a single, different, unmasked observer. Three corneal thickness measurements were taken at 10, 30, 60 and 70mmHg during increments, and at each 10mmHg decrement.
2.3 Data Analysis
Data were analyzed by linear regression and paired, 2-tailed Student’s t-test, using Microsoft Excel™. Data are reported as mean values ± standard deviation. Differences were considered significant when p≤0.05.
3. Results
3.1 Measurement variability using the TonoVet®
No significant inter-operator difference was observed (p > 0.479, data not shown) when comparing TonoVet® readings obtained by different operators and no significant difference was observed when comparing readings from the right vs. left eyes; thus all data for right and left eyes and for operators were pooled in subsequent statistical analyses. There was a tendency for readings taken in the right eye to be closer to ‘true’ IOP than those taken in the left eye, however this was not significant (mean differences between TV reading and true IOP were −0.46 ±6.25 and −3.07 ± 4.23, for right and left eyes respectively).
3.2 Comparison of the TonoVet® and Goldmann Applanation Tonometer (Figures 1,2,and 3, Table I)
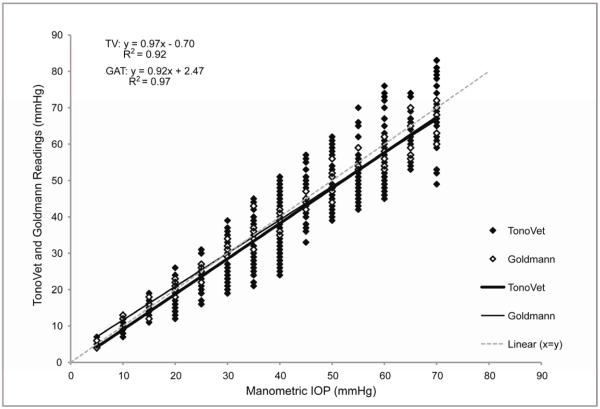
FIGURE 1.
Plot of all IOP readings taken with the TonoVet® and GAT (y) at each manometric IOP setting (x) in 3 monkeys, each contributing 2 eyes. TonoVet® readings taken by both operators and TonoVet® and GAT readings for both increments and decrements are presented. Some data points are superimposed.
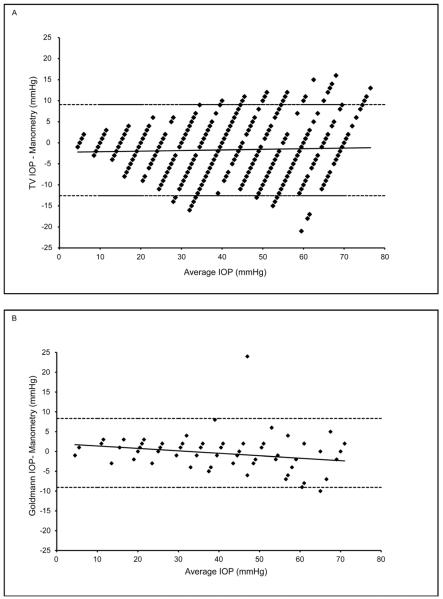
FIGURE 2.
Bland-Altman plots illustrating that the GAT has less variance but progressively greater deviation from true IOP (i.e. greater underestimation) at higher pressures, compared to the TonoVet®. A) IOP difference (TonoVet®−Manometric) plotted against average IOP ((TonoVet® + Manometric)/2). B) IOP difference (GAT−Manometric) against IOP average ((GAT+Manometric)/2). Dashed lines indicate 95% confidence intervals. Data are for 3 monkeys each contributing 2 eyes. Some data points are superimposed.
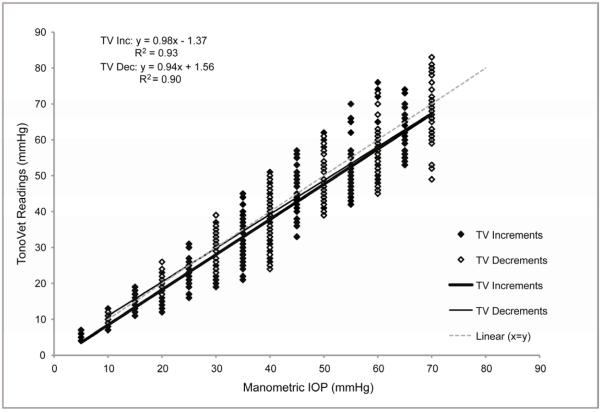
FIGURE 3.
Plot of Manometric IOP (x) versus TonoVet® IOP readings (y) obtained during increments (Inc) or decrements (Dec), with corresponding linear regression equations. Data are for 3 monkeys each contributing 2 eyes. Some data points are superimposed.
TABLE I.
Comparison of linear regression analyses for TV and GAT readings in three monkeys
| Slope | y-intercept | r2 coefficient | ||||
|---|---|---|---|---|---|---|
| TonoVet® | GAT | TonoVet® | GAT | TonoVet® | GAT | |
| Animal 1 | 1.082 | 0.988 | −0.101 | 2.033 | 0.961 | 0.987 |
| Animal 2 | 0.931 | 0.881 | −0.784 | 1.915 | 0.951 | 0.986 |
| Animal 3 | 0.913 | 0.887 | −1.666 | 3.250 | 0.954 | 0.978 |
| Mean | 0.975 | 0.919 | −0.850 | 2.399 | 0.955 | 0.984 |
| SD | 0.092 | 0.060 | 0.784 | 0.739 | 0.005 | 0.005 |
| p value | 0.103 | 0.062 | 0.017 | |||
Both the TonoVet® and the GAT readings demonstrated strong linear correlation with manometric IOP, as seen in Figure 1. The TV was highly accurate, and was comparable to GAT. There was no significant difference when comparing TonoVet® and GAT trend lines within individual animals (mean slopes = 0.975 and 0.919 respectively; p>0.102). There was a tendency for the TonoVet® to slightly underestimate IOP (−0.85±0.78mmHg); although this was not significant, it was consistent across the range of IOP. The GAT had less variance but progressively greater deviation from true IOP at higher pressures, compared to the TonoVet®. For both the TonoVet® and GAT, no significant differences were noted when comparing the device accuracy at lower IOP (5-25mmHg) and higher IOP (30-70mmHg) ranges. (Figures 1 and 2) TonoVet and GAT readings had comparable accuracy as manometric IOP was both increased and decreased (Figure 2). However, a slight, but statistically significant difference was observed when comparing the y-intercept of trend lines (p < 0.004), showing that TonoVet® IOP readings obtained during decrements were closer to true IOP when compared to those taken during increments (Figure 3). Similarly, the TonoVet® tended to underestimate IOP to a greater degree as IOP was increased, than when decreased (mean differences between manometric IOP and TonoVet® readings were 2.13 and 0.95mmHg, for incremental and decremental phases of the experiment respectively). Readings obtained with the GAT were slightly, but significantly more reproducible, and thus more precise, than those obtained with the TonoVet® [r2 coefficients = 0.98±0.01 (GAT) vs. 0.95±0.01 (TonoVet®); p=0.017] (Table I).
3.3 Corneal Thickness Measurements (Figure 4)
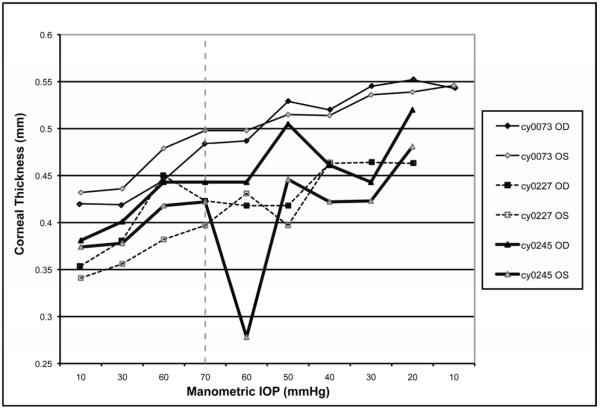
FIGURE 4.
Corneal thickness in mm (y) vs. manometric IOP in mmHg (x) for both increments (shown to the left of the dotted line) and decrements (shown to the right of the dotted line).
Central corneal thickness increased over the course of the experiment in all six eyes. Mean central corneal thickness was significantly greater at the conclusion of the experiment when compared to the mean central corneal thickness at the beginning of the experiment (0.511±0.017mm vs. 0.384±0.015mm, respectively; p<0.001).
4. DISCUSSION
In the current study, a strong correlation was found between IOP readings obtained manometrically and those obtained with the TonoVet® in cynomolgus macaques. This finding is consistent with the results reported by Yu et. al. (2009), in a paper that validated the accuracy of the TonoVet® in rhesus macaques, a distinct but related species with a larger eye, when compared to manometry. We also observed that GAT readings were significantly more precise or reproducible than TonoVet® readings when compared to manometry. In the current study, we found that the degree of precision or reproducibility for the TonoVet® (r2=0.95) in cynomolgus macaques, overall, was comparable to that reported previously for rhesus macaques (r2=0.97) (Yu et. al., 2009). In the current study, no significant differences were found in the accuracy or precision of the TonoVet® when comparing lower vs. higher IOPs. There was, however, an apparent increase in the variance of TonoVet® values as manometric IOP was increased. Since the TonoVet® is relatively easy and quick to use, it is recommended that multiple readings be obtained in order to account for any outliers or inconsistent values. The accuracy and precision determined for the TonoVet® in the current study compare very favorably to those determined for the Tono-Pen XL™ hand-held applanation tonometer by similar experiments conducted in this species in our laboratory. Previously we found that the Tono-Pen XL™, although precise, was relatively inaccurate in the cynomolgus macaque (slope = 0.692±0.016, intercept=1.21±0.60, correlation coefficient = 0.98) (Peterson et. al., 1996). On the basis of our findings, the TonoVet® appears to represent a more appropriate choice of instrument for measuring IOP in the cynomolgus macaque than the Tono-Pen XL™.
The current study showed a tendency for the TonoVet® to underestimate IOP in cynomolgus macaques, relative to manometry (mean difference = −1.72 ± 5.5mmHg), however this was not significant. This tendency could be due to several factors including corneal biomechanical properties, such as thickness and hysteresis. In our study, mean central corneal thickness was significantly greater at the end of the experiment compared to that measured at the beginning of the experiment. Several factors may have contributed to the observed increase in corneal thickness over the course of our experiments. These factors include, but are not limited to, the following: corneal exposure and desiccation of the tear film and corneal epithelium; effects of relatively rapid changes in hydrostatic pressure on the corneal endothelium and hydration of the stroma (Harris et. al., 1956; Kinsey and Cogan, 1942; Mayes and Hodson, 1978; Svedbergh, 1975; Ytteborg and Dohlman, 1965;), mechanical damage to the corneal epithelium as a result of repeated IOP measurements, and/or toxic effects of repeated topical applications of Fluress™ (0.4 % benoxinate HCl + 0.25% fluorescein sodium) for GAT measurements. Topical anesthetics such as 0.4% benoxinate may affect the pre-corneal tear film stability, health of the epithelium, and thickness of the cornea. (Asensio et. al, 2003; Yeung et. al., 2000). (Nam et. al., 2006) (McGee and Fraunfelder, 2007; Rosenwasser et. al., 1990). (McGee and Fraunfelder, 2007; Rosenwasser et. al., 1990). It is unlikely that the increase in corneal thickness found in our study was due to diurnal variation. Previous studies have found that maximum corneal thickness typically occurs upon waking and decreases throughout the day (Giráldez-Fernández et. al., 2008; Harper et. al., 1996; Read and Collins, 2009). Because our monkeys were under anesthesia, one could argue that a gradual thickening of the cornea took place over the course of the experiment, similar to what occurs during normal sleep. However, in our study the duration of each procedure was only 2-3 hours and not the typical 8-10 hour sleep interval. Additionally, previous studies have reported the mean overnight variation in central corneal thickness in humans with normal eyes to be between ~3.0 – 5.5% and overall maximal mean diurnal variation to be ~7% (Fogagnolo et. al., 2010; Harper et. al., 1996) thus it seems doubtful that the ~25% increase observed in the present study was due solely to diurnal variability in corneal thickness.
The TonoVet® underestimated IOP to a lesser extent during the decremental phase of the experiment, which also correlated with increasing corneal thickness. This finding supports previous studies indicating that corneal thickness in normal eyes plays a significant role in the accuracy of the TonoVet® in other species (Chui et. al., 2008; Iliev et. al., 2006; Kalesnykas and Uusitalo, 2007; Martinez-de-la-Casa et. al., 2006; Poostchi et. al., 2009). However, we cannot exclude the possibility that other corneal biomechanical properties, such as hysteresis, may have also changed during the course of the experiment and may have subtly influenced the accuracy of TonoVet® readings (Chui et. al., 2008; Jorge et. al., 2008). Based on previous work one would expect the TonoVet® to overestimate IOP as central corneal thickness increased (Tonnu et. al., 2005). As the normal corneal thickness of our cynomolgus macaques (0.384±0.015mm) is less than that of canines (0.562±0.062mm) (Gilger et. al., 1991), for which this tonometer has reportedly been calibrated, systematic underestimation of true IOP by the TonoVet® in this monkey species might be anticipated. In contrast to our findings, a previous study conducted in non-human primates by Yu et. al. (2009) reported a slight, but significant tendency for the TonoVet® to overestimate IOP at low manometric IOP values and to underestimate IOP at high manometric IOP values in rhesus macaques. This discrepancy could be due to several factors, including differences in methodology, inter-operator variability, or species differences between cynomolgus and rhesus macaques. It is recognized that inter-species differences in corneal biomechanical properties can result in differing accuracy and precision of tonometric readings (Reuter et. al., 2010). Rhesus macaques tend to have larger eyes as evidenced by greater axial length, vitreous chamber depth, anterior chamber depth (Crawford et. al., 1990; Fernandes et. al., 2003; Greene, 1990; Kaufman et. al., 1981) and somewhat thicker corneas (Jonas et. al., 2009; Madigan et. al., 1987; Okka et. al., 2004; Ollivier et. al., 2003; Peterson et. al., 2000; Tian et. al., 2001; Tian et. al., 2004). Since the GAT has some limitations in cases with corneal disease, such as edema, scarring, and/or corneal surface irregularities (Whitacre and Stein, 1993), it would be important to determine whether the TonoVet® would present an alternative method for measuring IOP in these situations with altered corneal biomechanical properties.
It is conceivable that the presence of the needle in the anterior chamber may have slightly altered the corneal shape in our subjects. This, in turn, may have affected IOP measurements since both the TonoVet® and GAT measure IOP by corneal contact. However, great care was taken during cannulation to place the needles in the inferior part of the anterior chamber, away from the axial area of tonometer contact, and to avoid gross corneal distortion. Any alteration in the corneal topography associated with cannulation would have been consistent throughout the experiment, and present during measurements obtained with both GAT and TonoVet®.
In the current study, we observed that TonoVet® readings obtained from the right eye had a tendency to be closer to true IOP, though this was not significant. Both operators did note that it was easier to position the TonoVet® so that the probe was directed horizontally, perpendicular to the axial corneal surface, when taking measurements in the right as compared to the left eyes, due to the facial conformation of the subjects. The small difference in accuracy between eyes could also have been attributable to the order in which IOPs were taken, since IOP was consistently measured in the right eye first throughout the study. Nonetheless, attention to consistent technique and proper positioning of the TonoVet® is still warranted since the angle and distance of the probe to the corneal surface can significantly change the reading obtained. In early experiments to validate rebound tonometry, Kontiola (2000) showed greater variability in IOP measurements when using a handheld rebound tonometer vs. a fixed rebound tonometer. Although no significant inter-operator variability was observed for the TonoVet® in this study, it is important to note that both of the TonoVet® operators in this experiment were right-handed and that the discrepancy between TonoVet® readings from the right and left eyes may prove to be different if operators are left-handed. Future studies comparing different TonoVet® units, left-handed vs. right-handed operators, and examining the potential influence of the order of ocular testing should be considered.
5. CONCLUSIONS
Based on the results of our study, the TonoVet® appears to offer a reproducible and practical method of monitoring IOP in cynomolgus monkeys with normal eyes, with accuracy comparable to the GAT. Further studies evaluating the use of the TonoVet® for IOP measurements in glaucomatous eyes and/or comparing it to other IOP measurement techniques such as pneumatonometry may also prove useful. The following conversion equation can be used to provide a more accurate estimate of true IOP: y = (1.028x + 0.721), where y is the estimation of true IOP and x is the IOP value obtained with the TV.
ACKNOWLEDGMENTS
Funding/Support: NIH Grants K08 EY018609 (GJM), EY02698 (PLK), P30 EY0016665 (PLK), Merck Merial Summer Scholarship (EJE), and the Wisconsin National Primate Research Center, University of Wisconsin-Madison, funded by Base Grant 5P51 RR 000167
The authors would like to thank Elizabeth A. Hennes for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abraham LM, Epasinghe NCR, Selva D, Casson R. Comparison of the ICare® rebound tonometer with the Goldmann applanation tonometer by experienced and inexperienced tonometrists. Eye (Basingstoke) 2008;22:503–506. doi: 10.1038/sj.eye.6702669. [DOI] [PubMed] [Google Scholar]
- Abrams LS, Vitale S, Jampel HD. Comparison of three tonometers for measuring intraocular pressure in rabbits. Invest. Ophthalmol. Vis. Sci. 1996;37:940–944. [PubMed] [Google Scholar]
- The AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS):7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Asensio I, Rahhal SM, Alonso L, Palanca-Sanfrancisco JM, Sanchis-Gimeno JA. Corneal thickness values before and after oxybuprocaine 0.4% eye drops. Cornea. 2003;22:527–532. doi: 10.1097/00003226-200308000-00008. [DOI] [PubMed] [Google Scholar]
- Bárány EH. Simultaneous measurements of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest. Ophthalmol. 1964;2:135–143. [PubMed] [Google Scholar]
- Bárány EH, Rohen JW. Localized contraction and relaxation within the ciliary muscle of the vervet monkey (Cercopithecus ethiops); The Structure of the Eye, Second Symposium; Stuttgart. 1965.pp. 287–311. [Google Scholar]
- Crawford KS, Kaufman PL, Bito LZ. The role of the iris in accommodation of rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 1990;31:2185–2190. [PubMed] [Google Scholar]
- Chui WS, Lam A, Chen D, Chiu R. The influence of cornea properties on rebound tonometry. Ophthalmol. 2008;115:80–84. doi: 10.1016/j.ophtha.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Eisenberg DL, Sherman BG, McKeown CA, Schuman JS. Tonometry in adults and children: A manometric evaluation of pneumatonometry, applanation, and TonoPen in vitro and in vivo. Ophthalmol. 1998;105:1173–1181. doi: 10.1016/S0161-6420(98)97016-6. [DOI] [PubMed] [Google Scholar]
- Fernandes A, Bradley DV, Tigges M, Tigges J, Herndon JG. Ocular measurements throughout the adult life span of rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 2003;44:2373–2380. doi: 10.1167/iovs.02-0944. [DOI] [PubMed] [Google Scholar]
- Filippopoulos T, Matsubara A, Danias J, et al. Predictability and limitations of non-invasive murine tonometry: Comparison of two devices. Exp. Eye Res. 2006;83:194–201. doi: 10.1016/j.exer.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Fogagnolo P, Capizzi F, Orzalesi N, Figus M, Ferreras A, Rossetti L. Can mean central corneal thickness and its 24-hour fluctuation influence fluctuation of intraocular pressure? J. Glaucoma. 2010;19:418–423. doi: 10.1097/IJG.0b013e3181aff432. [DOI] [PubMed] [Google Scholar]
- Gilger BC, Whitley RD, McLaughlin SA, Wright JC, Drane JW. Canine corneal thickness measured by ultrasonic pachymetry. Am. J. Vet. Res. 1991;52:1570–1572. [PubMed] [Google Scholar]
- Giráldez-Fernández MJ, Díaz-Rey A, García-Resua C, Yebra-Pimentel-Vilar E. Diurnal variations of central and paracentral corneal thickness and curvature. Arch. Soc. Esp. Oftalmol. 2008;83:183–192. doi: 10.4321/s0365-66912008000300010. [DOI] [PubMed] [Google Scholar]
- Gonnering RS, Dortzbach RK, Erickson KA, Kaufman PL. The cynomolgus monkey as a model for orbital research. I. Normal anatomy. Curr. Eye Res. 1983;3:529–540. doi: 10.3109/02713688409003053. [DOI] [PubMed] [Google Scholar]
- Görig C, Coenen RTI, Stades FC, Djajadiningrat-Laanen SC, Boevé MH. Comparison of the use of new handheld tonometers and established applanation tonometers in dogs. Am. J. Vet. Res. 2006;67:134–144. doi: 10.2460/ajvr.67.1.134. [DOI] [PubMed] [Google Scholar]
- Greene PR. Optical constants and dimensions for the myopic, hyperopic and normal rhesus eye. Exp. Eye Res. 1990;51:351–360. doi: 10.1016/0014-4835(90)90148-n. [DOI] [PubMed] [Google Scholar]
- Harper CL, Boulton ME, Bennett D, Marcyniuk B, Jarvis-Evans JH, Tullo AB, Ridgway AE. Diurnal variations in human corneal thickness. Br. J. Ophthalmol. 1996;80:1068–1072. doi: 10.1136/bjo.80.12.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE, Gehrsitz L, Gruber L. The hydration of the cornea. II. The effect of the intraocular pressure. Am. J. Ophthalmol. 1956;42:325–329. [PubMed] [Google Scholar]
- Iliev ME, Goldblum D, Katsoulis K, Amstutz C, Frueh B. Comparison of rebound tonometry with Goldmann applanation tonometry and correlation with central corneal thickness. Brit. J. Ophthalmol. 2006;90:833–835. doi: 10.1136/bjo.2005.089870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M, Kim Y, Yi N, et al. Comparison of the rebound tonometer (TonoVet) with the applanation tonometer (TonoPen XL) in normal Eurasian Eagle owls (Bubo bubo) Vet. Ophthalmol. 2007;10:376–379. doi: 10.1111/j.1463-5224.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Hayreh SS, Tao Y. Central corneal thickness and thickness of the lamina cribrosa and peripapilary sclera in monkeys. Arch. Ophthalmol. 2009;127:1395–1396. doi: 10.1001/archophthalmol.2009.243. [DOI] [PubMed] [Google Scholar]
- Jorge J, González-Méijome J, Queirós A, Fernandes P, Parafita M. Correlations between corneal biomechanical properties measured with the ocular response analyzer and ICare rebound tonometry. J. Glaucoma. 2008;17:442–448. doi: 10.1097/IJG.0b013e31815f52b8. [DOI] [PubMed] [Google Scholar]
- Kalesnykas G, Uusitalo H. Comparison of simultaneous readings of intraocular pressure in rabbits using Perkins handheld, Tono-Pen XL, and TonoVet tonometers. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007;245:761–762. doi: 10.1007/s00417-006-0470-8. [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Davis GE. “Minified” Goldmann applanating prism for tonometry in monkeys and humans. Arch. Ophthalmol. 1980;98:542–546. doi: 10.1001/archopht.1980.01020030538022. [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Calkins BT, Erickson KA. Ocular biometry of the cynomolgus monkey. Curr. Eye Res. 1981;1:307–309. doi: 10.3109/02713688108999452. [DOI] [PubMed] [Google Scholar]
- Kim CY, Kuehn MH, Anderson MG, Kwon YH. Intraocular pressure measurement in mice: a comparison between Goldmann and rebound tonometry. Eye (Basingstoke) 2007;21:1202–1209. doi: 10.1038/sj.eye.6702576. [DOI] [PubMed] [Google Scholar]
- Kinsey VE, Cogan DG. The cornea. IV. Hydration properties of the whole cornea. Arch. Ophthalmol. 1942;28:449–463. [Google Scholar]
- Knollinger AM, Croix NCI, Barrett PM, Miller PE. Evaluation of a rebound tonometer for measuring intraocular pressure in dogs and horses. J. Am. Vet. Med. Assoc. 2005;227:244–248. doi: 10.2460/javma.2005.227.244. [DOI] [PubMed] [Google Scholar]
- Kontiola A. A new electromechanical method for measuring intraocular pressure. Doc. Ophthalmol. 1996-1997;93:265–276. doi: 10.1007/BF02569066. [DOI] [PubMed] [Google Scholar]
- Kontiola AI. A new induction-based impact method for measuring intraocular pressure. Acta. Ophthalmol. Scand. 2000;78:142–145. doi: 10.1034/j.1600-0420.2000.078002142.x. [DOI] [PubMed] [Google Scholar]
- Lee PY, Podos SM, Serle JB, Camras CB, Severin CH. Intraocular pressure effects of multiple doses of drugs applied to glaucomatous monkey eyes. Arch. Ophthalmol. 1987;105:249–252. doi: 10.1001/archopht.1987.01060020103038. [DOI] [PubMed] [Google Scholar]
- Leiva M, Naranjo C, Pena MT. Comparison of the rebound tonometer (ICare®) to the applanation tonometer (Tonopen XL®) in normotensive dogs. Vet. Ophthalmol. 2006;9:17–21. doi: 10.1111/j.1463-5224.2005.00429.x. [DOI] [PubMed] [Google Scholar]
- Leske MC. The epidemiology of open-angle glaucoma: a review. Am. J. Epidemiol. 1983;139:429–440. doi: 10.1093/oxfordjournals.aje.a113626. [DOI] [PubMed] [Google Scholar]
- Madigan MC, Gillard-Crewther S, Kiely PM, Crewther DP, Brennan NA, Efron N, Holden BA. Corneal thickness changes following sleep and overnight contact lens wear in the primate (Macaca fascicularis) Curr. Eye Res. 1987;6:809–815. doi: 10.3109/02713688709034848. [DOI] [PubMed] [Google Scholar]
- Martinez-de-la-Casa JM, Garcia-Feijoo J, Vico E, et al. Effect of corneal thickness on dynamic contour, rebound, and Goldmann tonometry. Ophthalmol. 2006;113:2156–2162. doi: 10.1016/j.ophtha.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Mayes KR, Hodson S. Some effects of hydrostatic pressure on corneal hydration during specular microscopy. Exp. Eye Res. 1978;26:141–145. doi: 10.1016/0014-4835(78)90111-2. [DOI] [PubMed] [Google Scholar]
- McGee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin. Drug Saf. 2007;6:637–640. doi: 10.1517/14740338.6.6.637. [DOI] [PubMed] [Google Scholar]
- Nam SM, Lee HK, Kim EK, Seo KY. Comparison of corneal thickness after the instillation of topical anesthetics. Cornea. 2006;25:51–54. doi: 10.1097/01.ico.0000179929.97651.59. [DOI] [PubMed] [Google Scholar]
- Okka M, Tian B, Kaufman PL. Effect of low-dose latrunculin-B on anterior segment physiologic features in the monkey eye. Arch. Ophthalmol. 2004;122:1482–1488. doi: 10.1001/archopht.122.10.1482. [DOI] [PubMed] [Google Scholar]
- Ollivier FJ, Brooks DE, Komaromy AM, Kallberg ME, Andrew SE, Sapp HL, Sherwood MB, Dawson WW. Corneal thickness and endothelial cell density measured by non-contact specular microscopy and pachymetry in Rhesus macaques (Macaca mulatta) with laser-induced ocular hypertension. Exp. Eye Res. 2003;76:671–677. doi: 10.1016/s0014-4835(03)00055-1. [DOI] [PubMed] [Google Scholar]
- Peterson JA, Kiland JA, Croft MA, Kaufman PL. Intraocular pressure measurement in cynomolgus monkeys: Tono-Pen versus manometry. Invest. Ophthalmol. Vis. Sci. 1996;37:1197–1199. [PubMed] [Google Scholar]
- Peterson JA, Tian B, McLaren JW, Hubbard WC, Geiger B, Kaufman PL. Latrunculins’ effects on intraocular pressure, aqueous humor flow, and corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2000;41:1749–1758. [PubMed] [Google Scholar]
- Poostchi A, Mitchell R, Nicholas S, Purdie G, Wells A. The iCare tonometer: comparisons with Goldmann tonometry, and influence of central corneal thickness. Clin. Exp. Ophthalmol. 2009;37:687–691. doi: 10.1111/j.1442-9071.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassmussen CA, Kaufman PL. Primate glaucoma models. J. Glaucoma. 2005;14:311–314. doi: 10.1097/01.ijg.0000169409.01635.bc. [DOI] [PubMed] [Google Scholar]
- Read SA, Collins MJ. Diurnal variation of corneal shape and thickness. Optom Vis. Sci. 2009;86:170–180. doi: 10.1097/OPX.0b013e3181981b7e. [DOI] [PubMed] [Google Scholar]
- Reuter A, Müller K, Arndt G, Eule JC. Accuracy and reproducibility of the TonoVet rebound tonometer in birds of prey. Vet. Ophthalmol. 2010;13(Suppl. 1):80–85. doi: 10.1111/j.1463-5224.2010.00817.x. [DOI] [PubMed] [Google Scholar]
- Serle JB, Podos SM, Kitazawa Y, Wang RF. A comparative study of latanoprost (Xalatan) and isopropyl unoprostone (Rescula) in normal and glaucomatous monkey eyes. Jpn. J. Ophthalmol. 1998;42:95–100. doi: 10.1016/s0021-5155(97)00128-7. [DOI] [PubMed] [Google Scholar]
- Stoiber J, Fernandez V, Lamar PD, Hitzl W, Fantes F, Parel JM. Ex vivo evaluation of Tono-Pen and pneumotonometry in cat eyes. Ophthalmic Res. 2005;38:13–18. doi: 10.1159/000088492. [DOI] [PubMed] [Google Scholar]
- Tian B, Sabanay I, Peterson JA, Hubbard WC, Geiger B, Kaufman PL. Acute effects of H-7 on ciliary epithelium and corneal endothelium in monkey eyes. Curr. Eye Res. 2001;22:109–120. doi: 10.1076/ceyr.22.2.109.5529. [DOI] [PubMed] [Google Scholar]
- Tian B, Wang R, Podos SM, Kaufman PL. Effects of topical H-7 on outflow facility, intraocular pressure, and corneal thickness in monkeys. Arch. Ophthalmol. 2004;122:1171–1177. doi: 10.1001/archopht.122.8.1171. [DOI] [PubMed] [Google Scholar]
- Tonnu PA, Ho T, Newson T, et al. The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometry, non-contact tonometry, the Tono-Pen XL, and Goldmann applanation tonometry. Br. J. Ophthalmol. 2005;89:851–854. doi: 10.1136/bjo.2004.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Millar JC, Pang IH, Wax MB, Clark AF. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest. Ophthalmol. Vis. Sci. 2005;46:4617–4621. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Lindsey JD. The importance of models in glaucoma research. J. Glaucoma. 2005;14:302–304. doi: 10.1097/01.ijg.0000169395.47921.02. [DOI] [PubMed] [Google Scholar]
- Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv. Ophthalmol. 1993;38:1–30. doi: 10.1016/0039-6257(93)90053-a. [DOI] [PubMed] [Google Scholar]
- Yeung KK, Kageyama JY, Carnevali T. A comparison of fluoracaine and fluorox on corneal epithelial cell desquamation after Goldmann applanation tonometry. J. Am. Optom. Assoc. 2000;71:49–54. [PubMed] [Google Scholar]
- Ytteborg J, Dohlman CH. Corneal edema and intraocular pressure. I. Animal experiments. Arch. Ophthalomol. 1965;74:375–381. doi: 10.1001/archopht.1965.00970040377018. [DOI] [PubMed] [Google Scholar]
- Yu W, Cao G, Qiu J, et al. Evaluation of monkey intraocular pressure by rebound tonometer. Mol Vis. 2009;15:2196–2201. [PMC free article] [PubMed] [Google Scholar]