Abstract
Animal studies have revealed the association between stearoyl-CoA desaturase 1 (SCD1) and obesity and insulin resistance. However, only a few studies have been undertaken in humans. We studied SCD1 in visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) from morbidly obese patients and their association with insulin resistance, sterol regulatory element binding protein-1 (SREBP-1) and ATPase p97, proteins involved in SCD1 synthesis and degradation. The insulin resistance was calculated in 40 morbidly obese patients and 11 overweight controls. Measurements were made of VAT and SAT SCD1, SREBP-1 and ATPase p97 mRNA expression and protein levels. VAT and SAT SCD1 mRNA expression levels in the morbidly obese patients were significantly lower than in the controls (P = 0.006), whereas SCD1 protein levels were significantly higher (P < 0.001). In the morbidly obese patients, the VAT SCD1 protein levels were decreased in patients with higher insulin resistance (P = 0.007). However, SAT SCD1 protein levels were increased in morbidly obese patients with higher insulin resistance (P < 0.05). Multiple linear regressions in the morbidly obese patients showed that the variable associated with the SCD1 protein levels in VAT was insulin resistance, and the variables associated with SCD1 protein levels in SAT were body mass index (BMI) and ATPase p97. In conclusion, these data suggest that the regulation of SCD1 is altered in individuals with morbid obesity and that the SCD1 protein has a different regulation in the two adipose tissues, as well as being closely linked to the degree of insulin resistance.
INTRODUCTION
The regulation of fatty acid–oxidation, fatty acid–synthesis and storage all play a vital role in the maintenance of normal intracellular lipid concentrations (1,2). Studies in mice have shown that stearoyl-CoA desaturase 1 (SCD1) may be a key piece in these processes (3). Recently, SCD1 was proposed to play a vital role in the explanation of obesity in Mediterranean countries (4). SCD1 is an endoplasmic reticulum-bound enzyme that converts different saturated fatty acids into monounsaturated fatty acids (5). The products of enzyme activity, monounsaturated fatty acids, regulate membrane fluidity, generate second messengers (6) and intervene in cell differentiation and apoptosis (7,8).
Experimental animal studies have revealed the association between SCD1 and obesity and insulin resistance (1,9). A high level of SCD1 favors the storage of fat (1). The reduced adiposity in SCD−/− mice has been attributed to reduced lipid synthesis and increased lipid oxygenation (10). Mice with a targeted disruption in the SCD1 gene are resistant to diet-induced weight gain and have increased insulin sensitivity compared with wild-type controls (7). Additionally, loss of SCD1 activity is associated with greater insulin sensitivity in skeletal muscle (11). However, a study in mice showed that SCD1 deficiency worsens the diabetes in obese mice (12). SCD1 also protects against palmitate-induced cell injury (13). However, total SCD1 deficiency represents an extreme phenotype that may not easily be compared with human physiology.
SCD1 is subject to transcription and enzyme activity regulation and an ubiquitin-protease–dependent degradation (14–16). Repression of the SCD1 gene expression by polyunsaturated fatty acids (PUFAs) has been shown to depend on sterol regulatory element binding protein-1 (SREBP-1). Besides, mRNA expression may differ according to the tissue examined (17,18). Different studies have shown the regulation of SCD1 synthesis and activity (14–18). However, to our knowledge, little is known about SCD1 degradation. Moreover, only one report has described the role of ATPase p97 in SCD1 degradation (16). ATPase p97 is a protein involved in the regulation of the proteasome-dependent degradation of SCD1. The influence of high insulin resistance on ATPase p97 expression is also unknown.
However, only a few studies have been undertaken in humans, with most of these aimed at evaluating the desaturation indexes calculated from the composition of serum fatty acids (10,19,20). These studies show discordant results concerning their relation with body mass index (BMI) and insulin resistance. A recent study showed that a high hepatic SCD1 activity index is associated with low liver fat content and high insulin sensitivity in obese participants (21). These data, together with those from previous animal studies (2,7,8,9,11,12), suggest that SCD1 may be involved in the regulation of insulin resistance in obesity.
In light of the possible association of SCD1 with obesity and insulin resistance and that, as far as we are aware, no one has yet undertaken a simultaneous study of SCD1 in visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) from morbidly obese patients, we aimed to (a) determine the differences between SCD1 in VAT and in SAT from morbidly obese patients; (b) evaluate the association of insulin resistance with SCD1 in VAT and SAT from morbidly obese patients; and (c) study the relation between SCD1 and SREBP-1 and ATPase p97, proteins involved in the regulation of the synthesis and proteasome-dependent degradation of SCD1, respectively.
MATERIALS AND METHODS
Subjects
The study included 40 morbidly obese individuals without type 2 diabetes mellitus and 11 controls who were overweight. The controls were selected with a similar BMI to the average BMI of the population they were taken from (27.5 ± 2.1 kg/m2) (22). An oral glucose tolerance test was performed in all patients to exclude the diabetic patients. None of the selected patients were diabetic or were receiving antilipidemic agents. The weight of all the individuals had been stable for at least 1 month. All the participants gave their informed consent, and the study was reviewed and approved by the Ethics and Research Committee of Carlos Haya Regional University Hospital, Malaga, Spain.
Laboratory Measurements
Blood samples were collected after a 12-h fast. The serum was separated and immediately frozen at −80°C. Serum biochemical parameters were measured in duplicate, as described (23). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from fasting insulin and glucose (24). From other studies, we have seen that insulin resistance may affect the expression of different genes (12,25,26). Therefore, the morbidly obese patients were classified into two groups (25): nondiabetic morbidly obese with low insulin resistance (HOMA-IR <4.7) (MO-low-IR) and non-diabetic morbidly obese with high insulin resistance (HOMA-IR ≥4.7) (MO-IR).
Adipose Tissue Fatty Acid Composition
Total lipids from frozen VAT and SAT samples were extracted with chloroform-methanol 2:1 (v/v). The triglycerides were separated by thin-layer chromatography on silica gel plates (Merck, Darmstadt, Germany) with hexane-ethylic ether-acetic acid (80:20:2, v/v/v) as the developing solvent. Total lipids and triglycerides were analyzed as described (27). SCD1 activity was calculated from total lipids as the ratio of 18:1(n-9)/18:0 and 16:1(n-7)/16:0 fatty acids.
Adipose Tissue mRNA
VAT and SAT were obtained in the fasting state from the morbidly obese patients during bariatric surgery and from the controls during surgery for hiatus hernia. The biopsy samples were washed in physiological saline and immediately frozen in liquid nitrogen. Biopsy samples were maintained at −80°C until analysis. Total RNA isolation was obtained using an RNeasy Lipid Tissue Mini Kit (Qiagen, Hamburg, Germany), as described (25). mRNA expression was measured by semi-quantitative real-time PCR. Gene expression was normalized to the housekeeping gene β-actin. Primers for the PCR were designed based on National Center for Biotechnology Information (NCBI) database sequences and obtained from Proligo (Sigma-Aldrich, St. Louis, MO) (Table 1).
Table 1.
Primers used for amplification.
| Gene | NCBI database sequences | Sense primers | Antisense primers |
|---|---|---|---|
| SCD1 | NM_005063 | 5′-CAGTGTGTTCGTTGCCACTT-3′ | 5′-GGTAGTTGTGGAAGCCCTCA-3′ |
| ATPase p97 | NM-007126.3 | 5′GACTCTGCTGAAGGGTCTGG3′ | 5′ATCCGTGAATCCATCGAGAG3′ |
| SREBP-1 | NM-001005291 | 5′-TGCATTTTCTGACACGCTTC-3′ | 5′-CCAAGCTGTACAGGCTCTCC-3′ |
| β-Actin | NM_001101 | 5′-TACAGCTTCACCACCACGGC-3′ | 5′-AAGGAAGGCTGGAAGAGTGC-3′ |
Expression levels were quantified in arbitrary units using a five-point serially diluted cDNA standard curve run at the same time as the samples. SCD1, SREBP-1 (also called SREBF1), ATPase p97 (also called valosin-containing protein [VCD]) and β-actin standards were generated by PCR amplification from control samples. Each reaction was run in duplicate and contained 2 μL cDNA template, 6.4 μL Syber water, 10 μL Syber-Green and 0.5 μmol/L of primers, giving a final reaction volume of 20 μL. Cycling parameters were 95°C for 15 min and then 30–40 cycles of 95°C for 15 sec, annealing temperature for 30 sec (SCD1: 55°C, SREBP-1: 60°C, ATPase p97: 62°C and β-actin: 60.4°C) and a final extension step of 72°C for 30 sec in which fluorescence was acquired. Melting curve analyses were performed to ensure only a single product was amplified. There is no variation in the expression of this housekeeping gene (β-actin) in the condition tested. Once the absolute values for SCD1, SREBP-1, ATPase p97 and β-actin from each sample were obtained, the SCD1/β-actin, SREBP-1/β-actin and ATPase p97/β-actin ratio were calculated.
Adipose Tissue Proteins
Proteins were isolated from the adipose tissue as indicated in the protocol of NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL, USA). Nuclear and cytoplasmic fractions were separated according to the manufacturer’s instructions. SCD1, ATPase p97 and β-actin were analyzed in the cytoplasmic fraction. A 68-kDa band corresponding to the mature SREBP-1 was detected and quantified in the nuclear fraction. SCD1, SREBP-1, ATPase p97 and β-actin were detected by Western blotting using 10–20 μg protein fractionated in a 10% SDS-PAGE gel, followed by electrophoretic transfer to a polyvinylidence difluoride membrane at 15 V for 1 h (28). The membranes were blocked in Protein-Free Tween 20 Blocking Buffer (Pierce Biotechnology) overnight at 4°C. After washing with PBS + 0.05% Tween 20, membranes were incubated with monoclonal anti-SCD1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-p97 ATPase (PROGEN Biotechnik, Heidelberg, Germany) or anti–β-actin (Sigma-Aldrich, St. Louis, MO, USA) at a dilution of 1:800, 1:10 or 1:1000, respectively, for 1 h at room temperature. For SREBP-1, membranes were incubated with polyclonal anti–SREBP-1 (Santa Cruz Biotechnology) at a dilution of 1:500 overnight at room temperature. Membranes were washed and incubated with horseradish peroxidase–conjugated secondary antibody (Promega, Madison, WI, USA) at a dilution of 1:20000, 1:500, 1:1000 or 1:5000, respectively, for 1 h at room temperature. The proteins were visualized with SuperSignal® West Pico Chemiluminescent Substrate (Pierce Biotechnology) and quantified by an Auto-Chemi System (UVP, Upland, CA, USA) and the image acquisition analysis software Labworks 4.6 (UVP). The results were expressed as SCD1/β-actin, SREBP-1/β-actin and ATPase p97/β-actin ratios.
Statistical Analysis
The statistical analysis was done with SPSS (Version 11.5 for Windows; SPSS, Chicago, IL, USA). Comparison between the results of the different groups was made with the Student t test or with one-way analysis of variance (ANOVA), and the post hoc analysis was done with the Duncan multiple range test. The Pearson correlation coefficient was calculated to estimate the linear correlations between variables. Multiple linear regressions were used to determine the association between variables. Values were considered to be statistically significant when P ≤ 0.05. The results are given as the mean ± SD.
RESULTS
Anthropometric and Biochemical Characteristics
Table 2 summarizes the characteristics of the controls and the morbidly obese patients. Anthropometric data (weight, BMI and waist and hip circumferences) and serum free fatty acids and leptin were, as expected, significantly greater in the morbidly obese patients, although no significant differences were seen between the two groups of morbidly obese patients. No significant differences were found between the three groups of patients in serum glucose, cholesterol or triglycerides. Serum insulin and HOMA-IR in the morbidly obese patients with high insulin resistance were significantly higher than in those morbidly obese patients with low insulin resistance and the controls. Serum alanine aminotransferase in the morbidly obese patients with high insulin resistance was significantly higher than in those morbidly obese patients with low insulin resistance. Serum γ-glutamyltransferase in the morbidly obese patients with high insulin resistance was significantly higher than in controls, but was similar to morbidly obese patients with low insulin resistance. Serum adiponectin was significantly lower in the morbidly obese patients with high insulin resistance.
Table 2.
Anthropometric and biochemical variables in the morbidly obese patients, classified according to their HOMA-IR, and the controls.
| Controls | MO-low-IR | MO-IR | |
|---|---|---|---|
| n (men/women) | 11 (3/8) | 22 (6/16) | 18 (6/12) |
| Age (years) | 49.2 ± 15.4 | 43.6 ± 13.4 | 41.0 ± 8.8 |
| Weight (kg) | 77.0 ± 13.0 | 145.2 ± 30.8a | 158.8 ± 24.8a |
| BMI (kg/m2) | 27.5 ± 2.1 | 54.5 ± 7.3a | 56.1 ± 5.9a |
| Waist (cm) | 84.8 ± 8.7 | 139.4 ± 20.6a | 150.6 ± 19.5a |
| Hip (cm) | 103.2 ± 7.4 | 154.9 ± 18.7a | 157.5 ± 13.1a |
| Waist-to-hip ratio | 0.88 ± 0.08 | 0.89 ± 0.06 | 0.95 ± 0.11 |
| Glucose (mmol/L) | 6.04 ± 0.60 | 5.47 ± 0.53 | 5.82 ± 0.65 |
| Cholesterol (mmol/L) | 4.87 ± 0.86 | 5.28 ± 1.08 | 4.86 ± 0.72 |
| Triglycerides (mmol/L) | 0.90 ± 0.41 | 1.28 ± 0.47 | 1.38 ± 0.85 |
| Free fatty acids (mmol/L) | 0.315 ± 0.030 | 0.739 ± 0.320a | 0.659 ± 0.234a |
| Insulin (pmol/L) | 76.7 ± 31.8 | 115.8 ± 28.2 | 280.9 ± 88.3a,b |
| HOMA-IR | 3.14 ± 1.43 | 3.90 ± 1.00 | 9.90 ± 2.90a,b |
| Aspartate aminotransferase (U/L) | 20.7 ± 8.3 | 20.1 ± 7.2 | 22.1 ± 7.6 |
| Alanine aminotransferase (U/L) | 42.2 ± 13.5 | 34.9 ± 12.0 | 48.2 ± 18.8b |
| γ-Glutamyltransferase (U/L) | 32.2 ± 17.0 | 54.5 ± 77.2 | 73.9 ± 110.0a |
| Leptin (ng/mL) | 10.0 ± 3.4 | 185.9 ± 97.6a | 189.7 ± 70.7a |
| Adiponectin (ng/mL) | 19.7 ± 8.27 | 11.0 ± 4.4a | 8.2 ± 4.17a,b |
The results are given as the mean ± SD. Comparison between the results of the three groups was made with one-way ANOVA, and the post hoc analysis was done with the Duncan multiple range test. Values were considered to be statistically significant when P ≤ 0.05.
Significant differences with the control group (P < 0.05).
Significant differences between the means of the 2 groups of morbidly obese patients (P < 0.05).
The VAT from the morbidly obese patients contained a significantly higher percentage of n-6 PUFAs (Figure 1A) and a lower percentage of saturated fatty acids in the triglycerides (24.6 ± 4.3 versus 32.0 ± 8.5, P = 0.024) than in the control group. The 18:1(n-9)/18:0 ratio in the total lipids of VAT and SAT from the morbidly obese patients was also significantly greater than that in the controls (VAT: 18.1 ± 4.6 versus 12.2 ± 4.0, P = 0.026; SAT: 17.3 ± 3.4 versus 11.5 ± 3.6, P = 0.004). The 16:1(n-7)/16:0 ratio in total lipids of VAT and SAT from the morbidly obese patients was greater than that in the controls, although not significantly (VAT: 0.23 ± 0.06 versus 0.19 ± 0.05, P = 0.345; SAT: 0.17 ± 0.04 versus 0.15 ± 0.05, P = 0.489). No significant differences were detected between the two groups of morbidly obese patients in percentages of fatty acids (data not shown) or in the SCD1 activity index in the VAT or SAT (Figure 1B, C). No significant differences were found in the fatty acid composition according to sex (data not shown).
Figure 1.
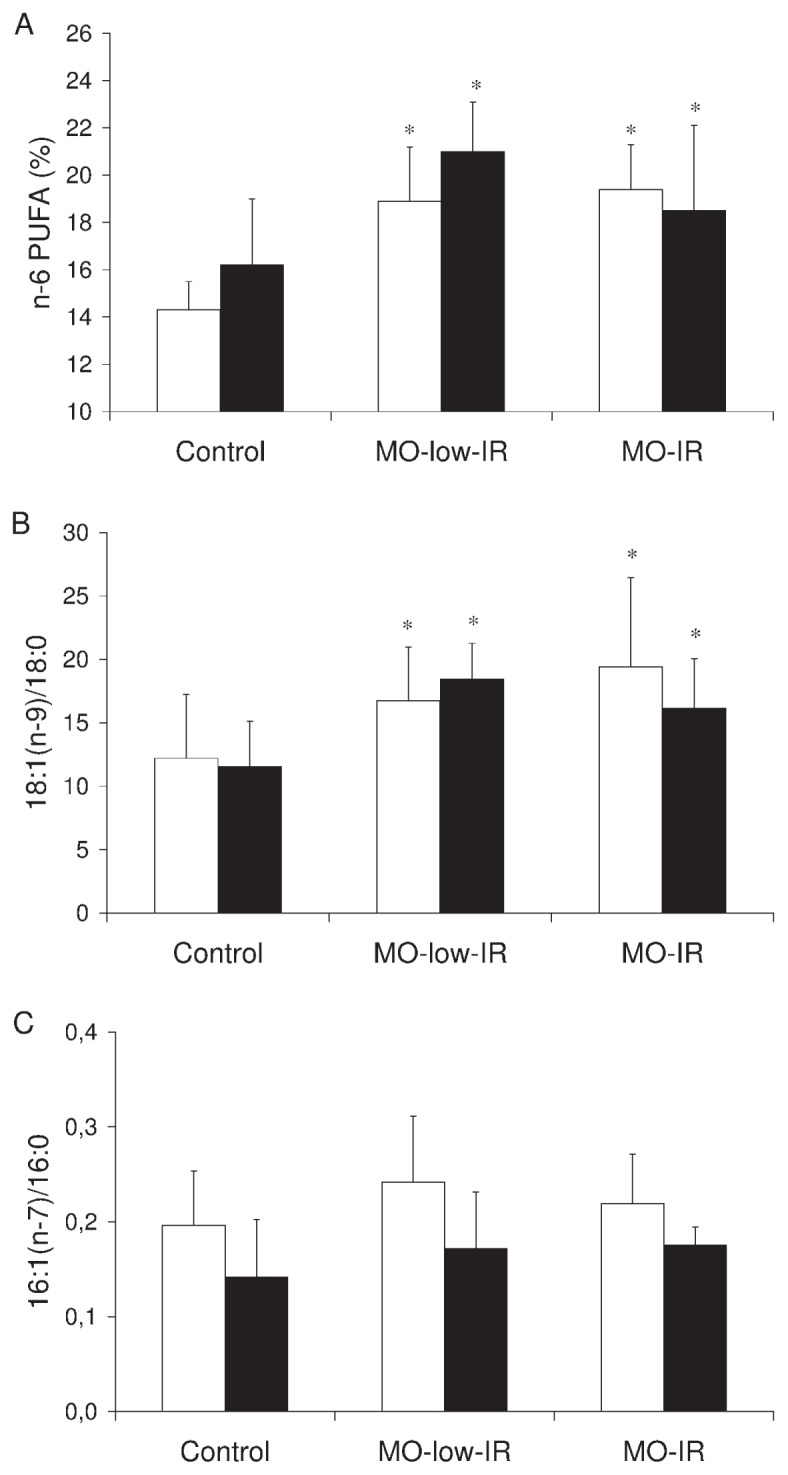
Fatty acid composition and SCD1 activity indexes. (A) n-6 PUFAs in the triglycerides in visceral and subcutaneous adipose tissue from controls and morbidly obese patients. (B) 18:1(n-9)/18:0 and (C) 16:1(n-7)/16:0 ratio in total lipids in VAT and SAT from controls and morbidly obese patients. White column, VAT; black column, SAT. The results are given as the mean ± SD. Comparison between the results of the 3 groups was made with one-way ANOVA. *Significant differences with the control group (P < 0.05).
SCD1 mRNA Expression and Protein Levels
The SCD1 mRNA expression in VAT and SAT from the controls was significantly greater than that from the morbidly obese patients (VAT: 0.153 ± 0.032 versus 0.049 ± 0.035, P = 0.006; SAT: 0.020 ± 0.004 versus 0.012 ± 0.005, P = 0.036). The expression in VAT from the morbidly obese patients and controls was significantly greater than that in SAT (morbidly obese patients: 0.049 ± 0.035 versus 0.012 ± 0.005, P = 0.001; controls: 0.153 ± 0.032 versus 0.020 ± 0.004, P = 0.001). No significant differences were detected between the two groups of morbidly obese patients in VAT (P = 0.287) or SAT SCD1 mRNA expression (P = 0.155) (Figure 2A). No significant differences were found in the SCD1 mRNA levels according to sex (data not shown).
Figure 2.
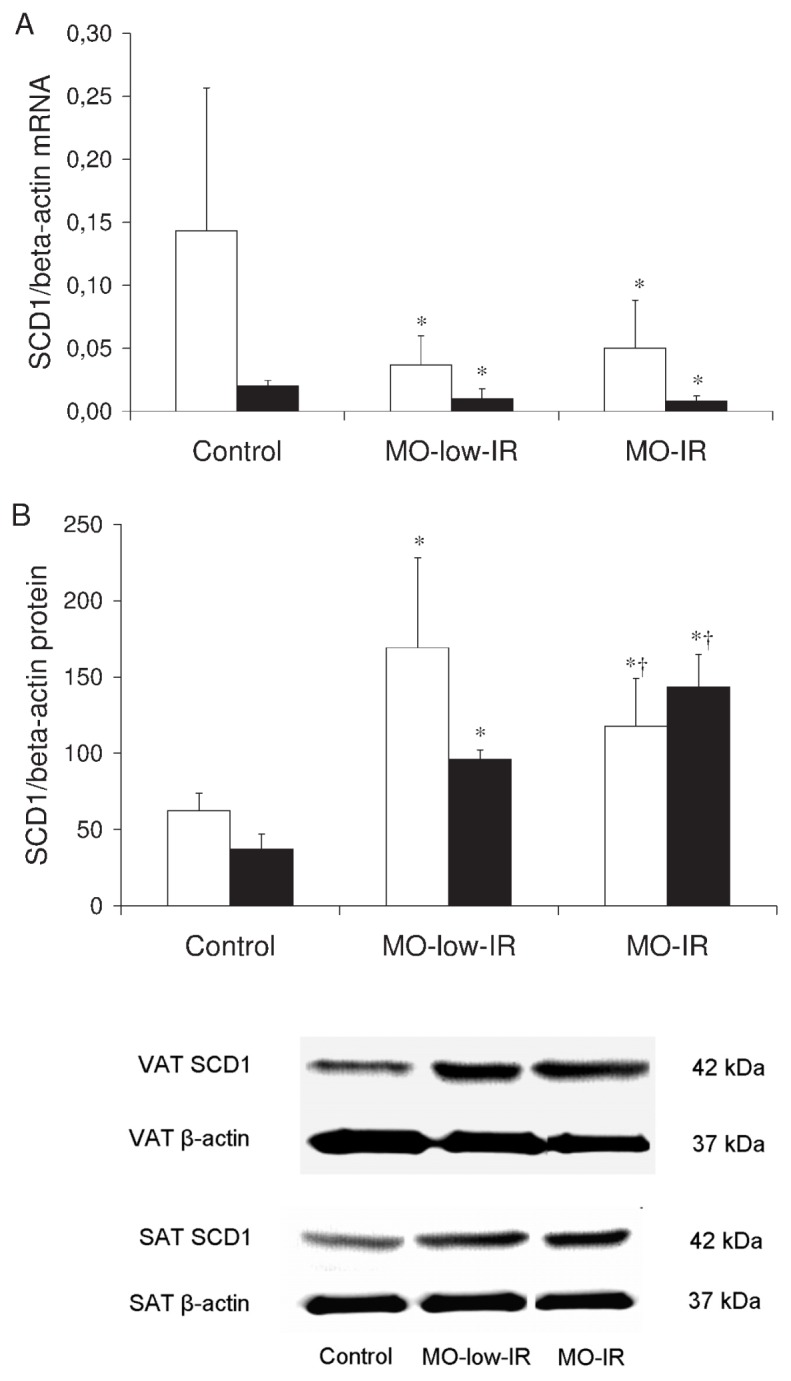
SCD1 in adipose tissue. (A) SCD1/β-actin mRNA expression levels in VAT and SAT from controls and morbidly obese patients. (B) SCD1/β-actin protein levels in VAT and SAT from controls and morbidly obese patients (n = 8 per group). White column, VAT; black column, SAT. The results are given as the mean ± SD. Comparison between the results of the 3 groups was made with one-way ANOVA. *Significant differences with the control group (P < 0.05). †Significant differences between the means of the 2 groups of morbidly obese patients (P < 0.05).
In VAT, the mRNA levels were associated in a multiple linear regression model with the BMI (P < 0.001) and SREBP-1 levels (r2 = 0.529, P = 0.041). When only the morbidly obese patients were considered, the VAT SCD1 mRNA expression levels correlated significantly with the SREBP-1 levels (r = 0.425, P = 0.006), after adjusting for BMI. The variable that was associated with the VAT SCD1 mRNA expression levels in a multiple linear regression model was the SREBP-1 level (r2 = 0.241, P = 0.034), after adjusting the model for age, BMI and HOMA-IR.
In SAT, the mRNA levels were associated in a multiple linear regression model with the BMI (P = 0.048) and the percentage of n-6 PUFAs in the SAT triglycerides (P = 0.043) (r2 = 0.730). When only the morbidly obese patients were considered, the SAT SCD1 mRNA expression levels correlated significantly with the percentage of n-6 PUFAs in the SAT triglycerides (r = −0.495, P = 0.038), after adjusting for BMI. The variables that were associated with the SCD1 mRNA expression levels in a multiple linear regression model were BMI (P = 0.022) and percentage of n-6 PUFAs in the SAT triglycerides (P = 0.011) (r = 0.862, r2 = 0.743), after adjusting the model for age, SREBP-1 levels and HOMA-IR.
However, the SCD1 protein levels in the VAT and SAT were significantly higher in the morbidly obese patients than the controls (VAT: 139.4 ± 56.2 versus 62.5 ± 11.3, P < 0.001; SAT: 119.4 ± 13.7 versus 37.1 ± 9.9, P < 0.001). In the VAT from morbidly obese patients, the MO-low-IR was the group that had higher SCD1 protein levels (P = 0.007) (Figure 2B). However, the SAT SCD1 protein levels were significantly higher in the MO-IR group (P = 0.005) (Figure 2B). No significant differences were found in the SCD1 protein levels according to sex (data not shown). VAT and SAT SCD1 protein levels in the morbidly obese patients correlated negatively (r = −0.604, P = 0.006).
In VAT, SCD1 correlated significantly with weight (r = 0.550, P = 0.001), BMI (r = 0.550, P = 0.001) and 18:1(n-9)/18:0 ratio (r = 0.521, P = 0.001). A multiple linear regression model showed that the VAT SCD1 protein levels were associated with BMI (P < 0.001) and HOMA-IR (P = 0.007) (r2 = 0.415). When only the morbidly obese patients were considered, the VAT SCD1 protein levels correlated significantly with HOMA-IR (r = −0.404, P = 0.030), after adjusting for BMI. HOMA-IR was also the variable that was associated with the VAT SCD1 protein levels in a multiple linear regression model (r2 = 0.158, P = 0.036), adjusting the model for age, BMI and ATPase p97 levels.
In SAT, the SCD1 protein levels were associated in a multiple linear regression model with the ATPase p97 levels (r2 = 0.849, P = 0.048). When only the morbidly obese patients were considered, the SAT SCD1 protein levels correlated significantly with HOMA-IR (r = 0.552, P = 0.022) and ATPase p97 levels (r = −0.823, P = 0.023), after adjusting for BMI. The variable that was associated with the SCD1 protein levels in a multiple linear regression model was the SAT ATPase p97 level (r2 = 0.526, P = 0.042), adjusting the model for age, BMI and HOMA-IR.
SREBP-1 mRNA Expression and Protein Levels
We determined whether the SCD1 mRNA expression levels were associated with the levels of SREBP-1. The expression in VAT from the morbidly obese patients and controls was significantly greater than that in SAT (morbidly obese patients: 0.0120 ± 0.0101 versus 0.0011 ± 0.0006, P = 0.001; controls: 0.027 ± 0.0172 versus 0.0020 ± 0.0014, P = 0.001). The SREBP-1 mRNA expression in VAT from the controls was significantly greater than that from the morbidly obese patients (0.027 ± 0.017 versus 0.012 ± 0.010, P = 0.014). No significant differences were detected between the two groups of morbidly obese patients in VAT (P = 0.416) (Figure 3A). No significant differences were detected between the three groups of patients in SAT (P = 0.526) (Figure 3A). No significant differences were found in the SREBP-1 mRNA levels according to sex (data not shown). These results were confirmed in VAT by Western blotting (Figure 3B). However, in SAT, the signal intensity measured using Western blotting was hardly detectable, with the results coinciding with those of the mRNA (data not shown).
Figure 3.
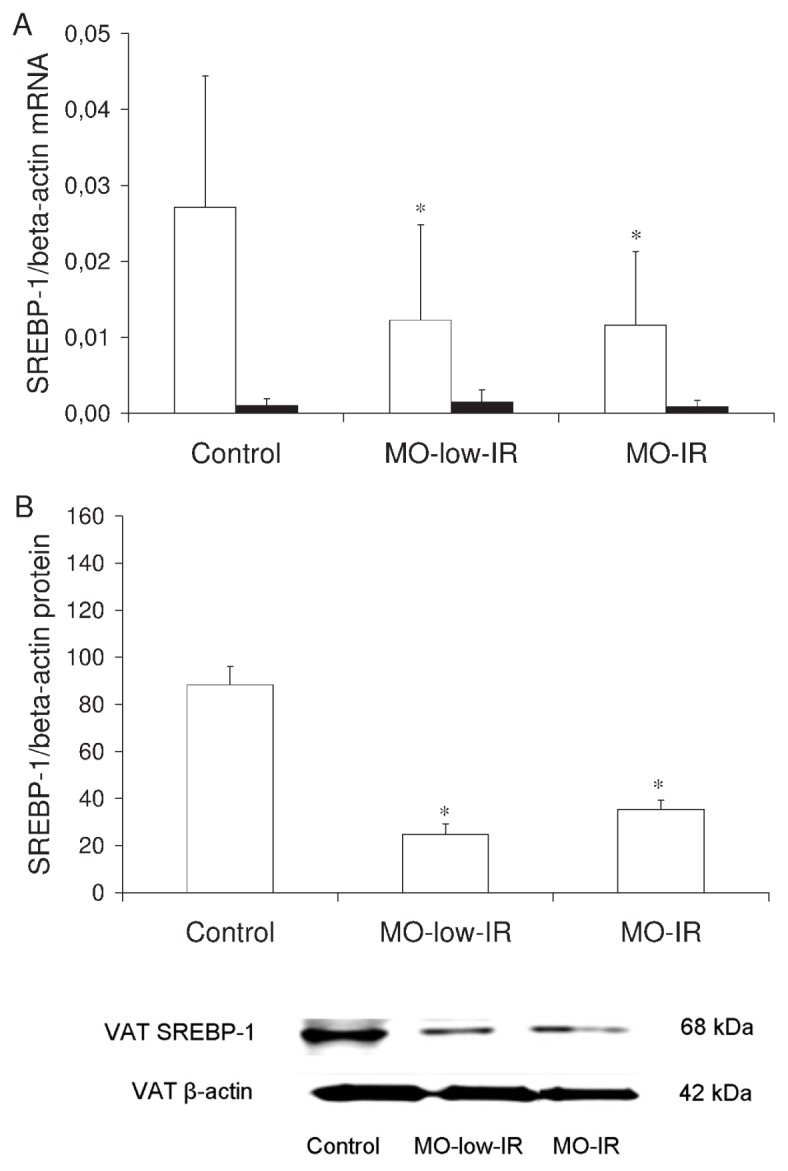
SREBP-1 in adipose tissue. (A) SREBP-1/β-actin mRNA expression levels in VAT and SAT from controls and morbidly obese patients. (B) SREBP-1/β-actin protein levels in VAT and SAT from controls and morbidly obese patients (n = 8 per group). White column, VAT; black column, SAT. The results are given as the mean ± SD. Comparison between the results of the 3 groups was made with one-way ANOVA. *Significant differences with the control group (P < 0.05).
In VAT, SREBP-1 correlated significantly with the percentage of n-6 PUFAs in the triglycerides (r = −0.387, P = 0.014) and with SCD1 mRNA (r = 0.471, P = 0.001). In a multiple linear regression model, the level of SREBP-1 was associated with the BMI (P = 0.026) and the percentage of n-6 PUFAs in the triglycerides (r2 = 0.254, P = 0.018), after adjusting the model for age and HOMA-IR. This association with the percentage of n-6 PUFAs in the triglycerides remained significant (r2 = 0.189, P = 0.022) when just the morbidly obese patients were considered.
In SAT, SREBP-1 correlated significantly with the HOMA-IR (r = −0.527, P = 0.010). In a multiple linear regression model, SREBP-1 was associated with HOMA-IR (r2 = 0.520, P = 0.034), after adjusting the model for age, BMI and the percentage of n-6 PUFAs in triglycerides. When only the morbidly obese patients were considered, SREBP-1 correlated significantly with HOMA-IR (r = −0.606, P = 0.008). In a multiple linear regression model, this association remained significant (r2 = 0.405, P = 0.048) after adjusting the model for age, BMI and the percentage of n-6 PUFAs in triglycerides.
ATPase p97 mRNA Expression and ATPase p97 Protein Levels
We also studied ATPase p97, an integral protein in the molecular complex that degrades SCD1. The ATPase p97 mRNA expression in VAT and SAT from the controls was similar to that from the morbidly obese patients (VAT: 0.037 ± 0.017 versus 0.036 ± 0.016, P = 0.774; SAT: 0.019 ± 0.009 versus 0.020 ± 0.013, P = 0.824). The expression in VAT from the morbidly obese patients and controls was significantly greater than that in SAT (morbidly obese patients: 0.036 ± 0.016 versus 0.020 ± 0.013, P = 0.001; controls: 0.037 ± 0.017 versus 0.019 ± 0.009, P = 0.001). However, in VAT from the morbidly obese patients, the MO-IR group had the highest ATPase p97 mRNA expression levels (P = 0.014) (Figure 4A). The SAT ATPase p97 mRNA expression levels were similar in the three groups of morbidly obese patients (Figure 4A). No significant differences were found in the SCD1 protein levels according to sex (data not shown). These results were confirmed by Western blotting (Figure 4B).
Figure 4.
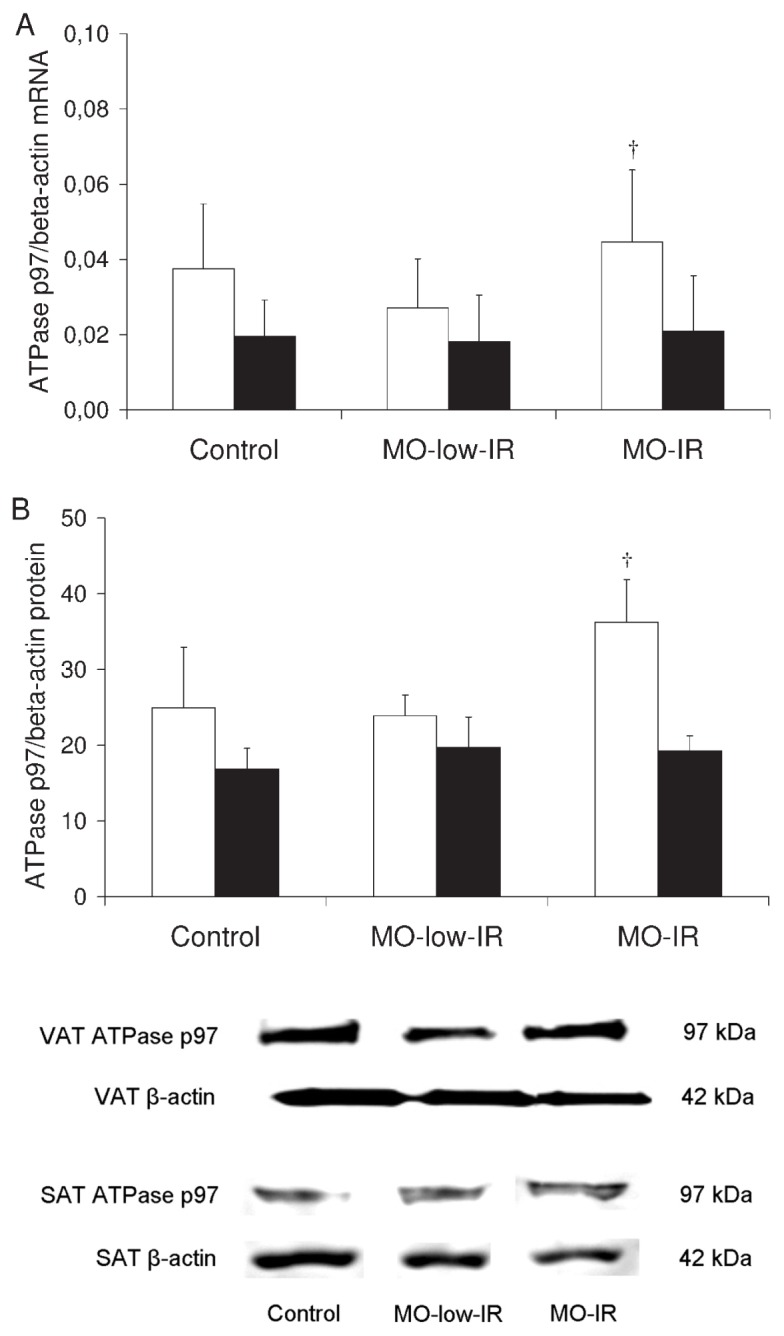
ATPase p97 in adipose tissue. (A) ATPase p97/β-actin mRNA expression levels in VAT and SAT from controls and morbidly obese patients. (B) ATPase p97/β-actin protein levels in VAT and SAT from controls and morbidly obese patients (n = 8 per group). White column, VAT; black column, SAT. The results are given as the mean ± SD. Comparison between the results of the 3 groups was made with one-way ANOVA. †Significant differences between the means of the 2 groups of morbidly obese patients (P < 0.05).
In VAT, ATPase p97 levels correlated with the HOMA-IR (r = 0.373, P = 0.006), but not with the SCD1 protein levels. This association remained significant (P = 0.014) (r2 = 0.155) after adjusting the multiple linear regression model for age, sex and BMI. When only the morbidly obese patients were considered, the correlation with HOMA-IR remained significant (r = 0.358, P = 0.015), even when the multiple linear regression model was adjusted for age, sex and BMI (r2 = 0.146, P = 0.017).
In SAT, the APTase p97 levels did not correlate significantly with the HOMA-IR, although they did correlate with the SAT SCD1 protein levels (r = −0.541, P = 0.046). When only the morbidly obese patients were considered, the correlation with SAT SCD1 protein levels remained significant (r = −0.725, P = 0.042).
DISCUSSION
The most relevant findings in this study are (a) the discrepancy between SCD1 mRNA expression and SCD1 protein level in VAT and SAT; (b) VAT and SAT SCD1 protein levels are clearly raised in morbidly obese patients; (c) VAT and SAT show different SCD1 mRNA expression and protein levels; (d) SCD1 mRNA expression levels are mainly associated with BMI, SREBP-1 levels and the percentage of n-6 PUFAs in triglycerides; and (e) SCD1 protein levels vary according to insulin resistance and ATPase p97 levels.
Our results show a relationship between SCD1 mRNA and BMI, with lower mRNA expression levels in VAT and SAT from morbidly obese patients than from controls. Lappalainen et al. (29) also showed that the expression of SCD1 in human SAT is decreased in obese subjects. Yao-Boren-gasser et al. (20) showed that there was no significant relationship between adipose SCD1 mRNA expression and BMI. However, Hulver et al. (19) showed that SCD1 is upregulated in skeletal muscle of obese people and is connected with lipid metabolism and insulin sensitivity. Moreover, our results also show lower mRNA expression levels in SAT from morbidly obese patients and controls than in VAT. Thus, SCD1 mRNA expression in humans may differ according to the tissue examined, as has been found in animals (18) and with other genes (17), and may suggest a different regulation of SCD1 mRNA expression in VAT and SAT. SCD1 mRNA expression is also highly regulated by fasting/refeeding. However, in our study, VAT and SAT samples were taken in the fasting state in all patients.
The associations found in our study between SCD1 mRNA expression and the n-6 PUFAs reflect the pretranslational regulation of SCD1 gene expression. The changes in mRNA stability are the most important factors affecting the abundance of SCD1 mRNA in adipocytes (14). mRNA levels were repressed by 80% of the original levels when 3T3-L1 adipocytes were exposed to 300 μmol/L arachidonic acid. Treatment of 3T3-L1 adipocytes with arachidonic acid also caused a threefold decrease in the half-life of SCD1 mRNA (14). n-6 PUFAs repress SCD1 gene expression by reducing the gene expression and maturation of SREBP-1 (8,14). As we saw, the n-6 PUFAs correlated negatively with SREBP-1, which was associated positively with SCD1 expression in VAT. In SAT, however, despite the presence of an association between the n-6 PUFAs and SCD1, this relation does not appear to be mediated by SREBP-1. Other transcription factors, such as Sp1, may be involved in the transcriptional regulation of SCD1 (30).
The SCD1 protein levels, however, were significantly raised in VAT and SAT from the morbidly obese patients, and they are associated with the 18:1(n-9)/18:0 activity index; 18:0 but not 16:0 is the preferred substrate of SCD1 in humans (31). The discrepancy between SCD1 mRNA expression and SCD1 protein level may be for reasons that fall outside the scope of this study. Nevertheless, studies with other proteins have shown that dissociation between mRNA and protein levels is common (32,33). Impaired regulation of SCD1 protein degradation in adipose tissue may also be involved in the increase found in this protein in morbidly obese individuals. SCD1 is a protein for which activity is regulated only by its concentration (34) and that is rapidly degradated (14). According to some studies, the amount of SCD1 protein does not depend just on mRNA expression (16,35). However, there is limited information on SCD1 protein degradation. A recent study suggested that SCD1 is degraded by proteasome-dependent endoplasmic reticulum–associated degradation (16). In SAT, our results show that the AT-Pase p97 protein level, which is essential for proteasome-dependent endoplasmic reticulum–associated degradation, was the variable that best explained the SCD1 protein level. Although the characteristics of this study impede determining whether ATPase p97 is the cause of the reduction in the SCD1 protein level, our results show a correlation that might suggest the involvement of the endoplasmic reticulum–associated degradation pathway in controlling SAT SCD1 (16). However, HOMA-IR is the main variable that is significantly associated with the SCD1 protein level in VAT, and it may be involved in its regulation. This index is a surrogate marker of insulin resistance, which partly reflects the insulin sensitivity. Nonetheless, other mechanisms must be involved in the explanation of SCD1 protein levels, such as an alteration in the posttranscriptional mRNA regulation. However, other experiments are needed to verify this hypothesis.
The mechanisms regulating obesity and insulin resistance are complex. Our results might suggest that SCD1 is an important factor in the control of obesity and insulin resistance, or perhaps a consequence. Previous studies have shown that SCD1 is associated with genes for which products are involved with lipid synthesis in adipocytes (20). The high level of SCD1 shown in morbidly obese subjects can favor the storage of fat (1). The idea is coming about that, in a positive energy balance, the amount of stored fat may be less important than the storage capacity of adipose tissue as a predictor of insulin resistance (36). Our results show that SCD1 protein levels in VAT were inversely associated with the insulin resistance. In the subjects with high insulin resistance, the VAT has lower levels of SCD1 protein and the SCD1 might increase in SAT, as shown by our results, or in muscle (10,19,37), favoring the storage of lipids. The accumulation of triglycerides in nonadipose tissues, such as muscle or liver, appears to be a measure of state associated with insulin resistance (38,39). Others have suggested that elevated SCD1 activity in skeletal muscle may represent a progression of the metabolic syndrome (19). However, Peter et al. (40) recently showed that high my-ocellular SCD1 activity protects from inflammation. Our results in visceral adipose tissue show that morbidly obese patients with low insulin resistance have high levels of SCD1 protein and a lower inflammatory response than individuals with high insulin resistance (41); in a previous study (41), we found that morbidly obese patients with high insulin resistance showed significantly elevated levels of tumor necrosis factor-α, interleukin (IL)-1β and IL-6 compared with individuals with low insulin resistance. Higher liver transaminases in morbidly obese patients with high insulin resistance might also suggest a different amount of fatty liver (42).
The underlying mechanisms for the different findings in VAT compared with SAT are unknown. The type of fat cells (adipocytes), their endocrine function, and response to insulin and other hormones differ between SAT and VAT. This might explain the tissue-specific dependent expression of SCD1. Also, VAT contains a greater number of large adipocytes in contrast to SAT, which contains small adipocytes. Small adipocytes are more insulin sensitive and have high avidity for free fatty acids and triglyceride uptake, preventing their deposition in non–adipose tissue (43,44).
In summary, we show that no concordance was found between SCD1 mRNA expression and the levels of SCD1 protein. The main conclusion is that SCD1 protein level is clearly raised in VAT and SAT from morbidly obese patients. However, a limitation of this study includes the small control group compared with the obese subjects and that we have no specific information regarding the dietary intake. Dietary intake plays an important role in the ratio of saturated to monounsaturated fatty acids. Experiments to analyze directly the SCD1 activity would give a more accurate measure of enzyme activity. In morbidly obese patients, SCD1 is associated with insulin resistance and could be considered a protective factor. The amount of VAT SCD1 protein was inversely related with the level of insulin resistance, whereas SAT SCD1 protein was directly related with the level of ATPase p97, a protein involved in its degradation. Like other studies (20,45), these results show that SCD1 in morbidly obese patients is related to obesity and insulin resistance. Nevertheless, the characteristics of this study impede determining whether the levels of SCD1 protein are a cause of obesity or insulin resistance or rather just a consequence. Additional studies in humans using different tissues should be carried out to show in-depth the mechanism by which SCD1 can be regulated in states of insulin resistance and in obesity.
ACKNOWLEDGMENTS
CIBER Fisiopatología de la Obesidad y Nutrición (CB06/03) and CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) are ISCIII projects. We thank Ian Johnstone for help with the English version of the text. This work was supported in part by grants from the Instituto de Salud Carlos III (CP04/00133, CP04/0039, PI07/0953 and PS09/01060) and Consejería de Salud de la Junta de Andalucía (0255/2007).
Footnotes
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Online address: http://www.molmed.org
REFERENCES
- 1.Dobrzyn A, Ntambi JM. The role of stearoyl-CoA desaturase in body weight regulation. Trends Cardiovasc Med. 2004;14:77–81. doi: 10.1016/j.tcm.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Dobrzyn P, et al. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci U S A. 2004;101:6409–14. doi: 10.1073/pnas.0401627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen P, Ntambi JM, Friedman JM. Stearoyl-CoA desaturase-1 and the metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:271–80. doi: 10.2174/1568008033340117. [DOI] [PubMed] [Google Scholar]
- 4.Soriguer F, et al. Obesity and the metabolic syndrome in Mediterranean countries: a hypothesis related to olive oil. Mol Nutr Food Res. 2007;51:1260–7. doi: 10.1002/mnfr.200700021. [DOI] [PubMed] [Google Scholar]
- 5.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturase and role in metabolism. Prog Lipid Res. 2004;43:91–104. doi: 10.1016/s0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 6.Gaster M, Rustan AC, Beck-Nielsen H. Differential utilization of saturated palmitate and unsaturated oleate: evidence from cultured my-otubes. Diabetes. 2005;54:648–56. doi: 10.2337/diabetes.54.3.648. [DOI] [PubMed] [Google Scholar]
- 7.Ntambi JM, et al. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–6. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999;40:1549–58. [PubMed] [Google Scholar]
- 9.Rahman SM, et al. Stearoyl-CoA desaturase 1 deficiency increases insulin signalling and glycogen accumulation in brown adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:381–7. doi: 10.1152/ajpendo.00314.2004. [DOI] [PubMed] [Google Scholar]
- 10.Sjögren P, et al. Fatty acid desaturases in human adipose tissue: relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia. 2008;51:328–35. doi: 10.1007/s00125-007-0876-9. [DOI] [PubMed] [Google Scholar]
- 11.Rahman SM, et al. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signalling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc Natl Acad Sci U S A. 2003;100:11110–5. doi: 10.1073/pnas.1934571100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flowers JB, et al. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007;56:1228–39. doi: 10.2337/db06-1142. [DOI] [PubMed] [Google Scholar]
- 13.Collins JM, Neville MJ, Hoppa MB, Frayn KN. De novo lipogenesis and stearoyl-CoA desaturase are coordinately regulated in the human adipocyte and protect against palmitate-induced cell injury. J Biol Chem. 2010;285:6044–52. doi: 10.1074/jbc.M109.053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sessler AM, Kaur N, Palta JP, Ntambi JM. Regulation of stearoyl-CoA desaturase 1 mRNA stability by polyunsaturated fatty acids in 3T3-L1 adipocytes. J Biol Chem. 1996;271:29854–8. doi: 10.1074/jbc.271.47.29854. [DOI] [PubMed] [Google Scholar]
- 15.Cohen P, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–3. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 16.Kato H, Sakaki K, Mihara K. Ubiquitin-proteasome-dependent degradation of mammalian ER stearoyl-CoA desaturase. J Cell Sci. 2006;119:2342–53. doi: 10.1242/jcs.02951. [DOI] [PubMed] [Google Scholar]
- 17.Carey AL, et al. Discordant gene expression in skeletal muscle and adipose tissue of patients with type 2 diabetes: effect of interleukin-6 infusion. Diabetologia. 2006;49:1000–7. doi: 10.1007/s00125-006-0178-7. [DOI] [PubMed] [Google Scholar]
- 18.Dridi S, Taouis M, Gertler A, Decuypere E, Buyse J. The regulation of stearoyl-CoA desaturase gene expression is tissue specific in chickens. J Endocrinol. 2007;192:229–36. doi: 10.1677/JOE-06-0070. [DOI] [PubMed] [Google Scholar]
- 19.Hulver MW, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–61. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao-Borengasser A, et al. Stearoyl-coenzyme A desaturase 1 gene expression increases after pioglitazone treatment and is associated with peroxisomal proliferator-activated receptor-gamma responsiveness. J Clin Endocrinol Metab. 2008;93:4431–9. doi: 10.1210/jc.2008-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefan N, et al. Low hepatic stearoyl-CoA desaturase 1 activity is associated with fatty liver and insulin resistance in obese humans. Diabetologia. 2008;51:648–56. doi: 10.1007/s00125-008-0938-7. [DOI] [PubMed] [Google Scholar]
- 22.Soriguer F, et al. Incidence of type 2 diabetes in southern Spain (Pizarra Study) Eur J Clin Invest. 2008;38:126–33. doi: 10.1111/j.1365-2362.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 23.García-Fuentes E, et al. Morbidly obese individuals with impaired fasting glucose have a specific pattern of insulin secretion and sensitivity: effect of weight loss after bariatric surgery. Obes Surg. 2006;16:1179–88. doi: 10.1381/096089206778392383. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Fuentes E, et al. PPAR_ expression after a high-fat meal is associated with plasma superoxide dismutase activity in morbidly obese persons. Obesity (Silver Spring) 2010;18:952–8. doi: 10.1038/oby.2009.314. [DOI] [PubMed] [Google Scholar]
- 26.Tinahones FJ, et al. Obesity and insulin resistance-related changes in the expression of lipogenic and lipolytic genes in morbidly obese subjects. Obes Surg. 2010;20:1559–67. doi: 10.1007/s11695-010-0194-z. [DOI] [PubMed] [Google Scholar]
- 27.Soriguer F, et al. Changes in the serum composition of free-fatty acids during an intravenous glucose tolerance test. Obesity (Silver Spring) 2008;17:10–5. doi: 10.1038/oby.2008.475. [DOI] [PubMed] [Google Scholar]
- 28.Soriguer F, Garcia-Serrano S, Garrido-Sanchez L, Gutierrez-Repiso C, Rojo-Martinez G, et al. Jejunal wall triglyceride concentration of morbidly obese persons is lower in those with type 2 diabetes mellitus. J Lipid Res. 2010;51:3516–23. doi: 10.1194/jlr.M007815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lappalainen T, et al. Adipose tissue stearoyl-CoA desaturase 1 (SCD1) gene expression in obese subjects. Int J Obes. 2007;31:S46–77. [Google Scholar]
- 30.Mauvoisin D, Prévost M, Ducheix S, Arnaud MP, Mounier C. Key role of the ERK1/2 MAPK pathway in the transcriptional regulation of the stearoyl-CoA desaturase (SCD1) gene expression in response to leptin. Mol Cell Endocrinol. 2010;319:116–28. doi: 10.1016/j.mce.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Kim YC, Gomez FE, Fox BG, Ntambi JM. Differential regulation of the stearoyl-CoA desaturase genes by thiazolidinediones in 3T3-L1 adipocytes. J Lipid Res. 2000;41:1310–6. [PubMed] [Google Scholar]
- 32.Rasouli N, Yao-Borengasser A, Miles LM, Elbein SC, Kern PA. Increased plasma adiponectin in response to pioglitazone does not result from increased gene expression. Am J Physiol Endocrinol Metab. 2006;290:42–6. doi: 10.1152/ajpendo.00240.2005. [DOI] [PubMed] [Google Scholar]
- 33.Højbjerre L, Rosenzweig M, Dela F, Bruun JM, Stallknecht B. Acute exercise increases adipose tissue interstitial adiponectin concentration in healthy overweight and lean subjects. Eur J Endocrinol. 2007;157:613–23. doi: 10.1530/EJE-07-0213. [DOI] [PubMed] [Google Scholar]
- 34.Heinemann FS, Ozols J. Stearoyl-CoA desaturase, a short-lived protein of endoplasmic reticulum with multiple control mechanisms. Prostaglandins Leukot. Essent. Fatty Acids. 2003;68:123–33. doi: 10.1016/s0952-3278(02)00262-4. [DOI] [PubMed] [Google Scholar]
- 35.Braun S, Matuschewski S, Rape M. Role of the ubiquitin-selective CDC48 (UFD1/NPL4) chaperone (segregase) in ERAD of OLE1 and other substrate. EMBO J. 2002;21:615–21. doi: 10.1093/emboj/21.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medina-Gomez G, et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3:634–47. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinnamaneni SK, Southgate RJ, Febbraio MA, Watt MJ. Stearoyl CoA desaturase 1 is elevated in obesity but protects against fatty acid-induced skeletal muscle insulin resistance in vitro. Diabetologia. 2006;49:3027–37. doi: 10.1007/s00125-006-0427-9. [DOI] [PubMed] [Google Scholar]
- 38.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55(Suppl 2):S16–23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- 39.Kraegen EW, Cooney GJ. Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol. 2008;19:235–41. doi: 10.1097/01.mol.0000319118.44995.9a. [DOI] [PubMed] [Google Scholar]
- 40.Peter A, et al. Individual stearoyl-CoA desaturase 1 expression modulates endoplasmic reticulum stress and inflammation in human my-otubes and is associated with skeletal muscle lipid storage and insulin sensitivity in vivo. Diabetes. 2009;58:1757–65. doi: 10.2337/db09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbarroja N, et al. The obese healthy paradox: is inflammation the answer. Biochem J. 2010;430:141–9. doi: 10.1042/BJ20100285. [DOI] [PubMed] [Google Scholar]
- 42.Stefan N, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–16. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 43.Marin P, et al. The morphology and metabolism of intra-abdominal adipose tissue in men. Metabolism. 1992;41:1241–8. doi: 10.1016/0026-0495(92)90016-4. [DOI] [PubMed] [Google Scholar]
- 44.Hisra A, Vikram NK. Clinical and patho-physiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition. 2003;19:457–66. doi: 10.1016/s0899-9007(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–44. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]


