Abstract
We describe here a simple device for dielectrophoretic concentration of marine microalga Karenia brevis non-motile cells, followed by electric field-mediated lysis for RNA extraction. The lysate was purified using magnetic beads and pure RNA extracted. RNA quality was assessed off-chip by nucleic acid sequence-based amplification and the optimum conditions for lysis were determined. This procedure will form part of an integrated microfluidic system that is being developed with sub-systems for performing cell concentration and lysis, RNA extraction/purification and real-time quantitative RNA detection. The integrated system and its components could be used for a large range of applications including in situ harmful algal bloom detection, transcriptomics and point-of-care diagnostics.
Keywords: electroporation, dielectrophoresis, Karenia brevis, RNA extraction, RNA amplification, dinoflagellate cysts
1. Introduction
Certain marine algae form harmful algal blooms (HABs) which are deleterious to ecosystems and can lead to major financial losses for fishery, tourism and healthcare industries, estimated at €584 millions in the European Union for 2005 [1]. Monitoring of HABs requires in situ sensors of high temporal and spatial resolution, reliability and accuracy that can withstand long-term deployment [2]. Typical deployment requirements are in the order of one year and critical factors are reagent consumption, power requirements and waste storage [3].
Studies have already shown that RNA amplification with nucleic acid sequence-based amplification (NASBA) is particularly suitable for early detection of Karenia brevis [4] and Karenia mikimotoi [5] on a macro-scale. NASBA is an isothermal process of nucleic acid amplification which occurs at 41°C, combines high amplification and rapid analysis and requires only the total lysate for analysis [6]. Hence, development of a miniaturized cell-lysis system would be both useful and profitable for NASBA-based lab-on-a-chip (LOC) seawater analysis systems. Microfluidic sample extraction [7,8], detection and analysis of nucleic acids [9–11] and optical detection systems for biosensor applications [12] have been thoroughly reviewed.
Cell lysis is defined as the disruption of cell membrane by physical, chemical, mechanical, thermal or enzymatic means in order to obtain intracellular materials [13]. Once cells are lysed, nucleic acids can be extracted and purified for further analyses using amplification methods such as polymerase chain reaction (PCR) for DNA, reverse transcriptase PCR (RT-PCR) or NASBA for RNA. Physical lysis includes osmotic shock and pressure; while mechanical methods rely on breakdown of cell membranes by shear and wear. A miniaturized mechanical lysis system with high efficiency has been demonstrated [14]. Detergents, solvents and antibiotics are used for chemical lysis in order to solubilize lipid membranes, both plasma and organelle. The chemical lysis method is the most commonly used in laboratories, with well-established bench-top protocols [15], which often include additional steps in order to remove excess surfactant from the total lysate. Micro-fluidic devices using this technique are available [16]. A combined physical and chemical lysis device employing electrochemically generated hydroxide ions acting as alkaline lytic agents has been shown [17]. Thermal lysis denatures proteins but leaves nucleic acids intact, and the Motorola Laboratories (USA) have developed a fully integrated chip using this method [18]. The state-of-the-art in lysis microfluidic devices has been recently reviewed [19] as well as single cell lysis on-chip [20,21] and general micro-electromechanical systems that include lysis steps [22,23].
In order to obtain all sub-cellular materials without the complications of chemical and mechanical lysis, we developed a simple electric field-based cell concentration and lysis method that could be incorporated within a complete microfluidic RNA extraction and amplification system. Cells are concentrated at electrodes using dielectrophoresis (DEP) [24], and then lysed using high strength electric field [25]. Electroporation of cell membranes was first presented in 1965 [26] and its theory has been reviewed [27]. Since then several micro-devices have been developed for electroporation. Electric field-mediated lysis was observed by microscopy for yeast and plant protoplast cells [28]. Human hepatocellular carcinoma cells transfected with green fluorescent protein genes were lysed on-chip by electroporation in a continuous flow microchip [29]. Single-cell electroporation of human prostate adenocarcinoma cells, reported by infiltration of YOYO-1 nucleic acid stain that cannot pass through intact cell membranes, has been demonstrated on-chip [30]. A micro-electroporation device was used to lyse human colon carcinoma cells, confirming lysis with a vital stain of acridine orange and propidium iodide (PI) [31]. Single plant protoplast cells as large as 85 µm were captured and lysed using two pairs of electrodes inside a pinched microchannel by applying an alternating voltage [32]. Pulsed discontinuous current lysed Bordella pertussis bacteria and lysis was validated by DNA recovery using real-time PCR [33]. Most recently, a similar device was described for the isolation and electroporation of A431 human epithelial carcinoma cells [34].
In this article, we have demonstrated concentration and electric field-mediated cell lysis of the phytoplankton K. brevis followed by extraction and amplification of RNA using bench-top NASBA methods. Generally, these cells are extremely difficult to lyse because they produce robust cysts. Cell lysis was confirmed by PI infiltration and by RNA yield, purity and quality based on absorbance spectrophotometry and amplification efficiency by NASBA.
2. Experimental methods
2.1. Cell concentration and electroporation
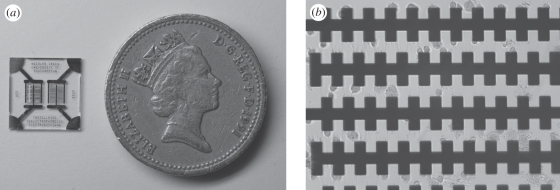
An array of interdigitated electrodes was used to both concentrate cells by DEP and subsequently perform electric field-mediated cell lysis. The micro-electrode chip consisted of a 3 × 4 mm (length × width) array of castellated interdigitated electrodes patterned on a 1 × 1 cm piece of glass. The electrodes were made of platinum with a thickness of 200 nm. The width and gap of micro-electrodes were 20 µm. Karenia brevis cells were attracted and trapped to the electrodes by positive DEP using an AC single phase of variable voltage and frequency. Lysis was performed using a high voltage, amplified with a Trimate AC generator (Model 1000A, Engler Engineering, USA). A miniaturized 3 × 4 × 3.5 mm (length × width × height) chamber was made from poly-methyl methacrylate (PMMA) and bonded to the microchip to hold the cell suspension.
2.2. Cell culture
The K. brevis cell strain was kindly donated by the Purdie laboratory at the National Oceanography Centre (Southampton, UK). The cells were grown in L1 Aquil* artificial seawater media at 20°C with 12 L : 12 D at high irradiance. Cell samples were harvested during exponential cell growth. Cell growth was monitored by counting 1 ml culture aliquots fixed in 1 per cent Lugol's solution (Sigma–Adrich, UK) in a Sedgwick-Rafter counting chamber (Fisher Scientific, UK). Cell cultures were used only if cells were larger than 20 µm on the day of the experiment.
2.3. Electric field-mediated lysis
A 1 ml aliquot of the cell culture was centrifuged at 20 000g for 1 min and the supernatant was discarded. The cell pellet was re-suspended in 1 ml of iso-osmotic low-conductivity buffer (280 mM mannose, 0.5% Tween, 10.5 mS m−1). The sample was centrifuged and re-suspended three times. The supernatant was discarded without mixing and the pellet was dissolved in 250 µl iso-osmotic low-conductivity buffer to a final cell density of approximately 300 000 cells ml−1. An aliquot of 42 µl of the iso-osmotic cell suspension was loaded into the PMMA chamber and cells were captured on the micro-electrodes by positive DEP (0.2 MHz, 1 V, 20 s). After the cells were trapped, the voltage and frequency was changed to perform cell lysis. The lysate was collected with a pipette and kept on ice. The process was repeated a further five times to process the 250 µl volume. The lysate was stored at −20°C for the NASBA process. To evaluate the efficiency of this method, the technique was compared with a commercial lysis protocol, which used lysis buffer and chemical extraction described in §2.4.
2.4. Assessment of lysis efficiency
Lysis conditions were optimized by observing the uptake of PI into the cells. PI was purchased from Sigma–Adrich, UK and has an excitation wavelength at 535 nm with emission at 617 nm, when DNA is bound to the dye. The PI solution at 50 µM was mixed into the iso-osmotic buffer and the cells observed with a fluorescence microscope during electroporation. Bright field observation of the cells was also conducted to image membrane damage. RNA from the cell lysate was purified using two commercial kits, one using magnetic beads (Nuclisens miniMAG, bioMérieux, The Netherlands) and another using a silica bead matrix (Rneasy kit, Qiagen, UK). The experimental protocols for both RNA extraction kits were followed according to the manufacture's guidelines. The quality of the pure RNA extract was detected using a Nanodrop UV–VIS spectrophotometer (Thermo Fisher, UK). The pure RNA extract was amplified and measured with a bench-top NASBA instrument (EasyQ analyser, bioMérieux, Netherlands). Lysis efficiency was validated against bench-top lysis using a commercial buffer containing chaotropic agent guanidine thiocyanate (Nuclisens Lysis Buffer, bioMérieux, The Netherlands). Conditions for NASBA reaction have been previously described [35]. Cell cultures were lysed on-chip and analysed by NASBA on the same day to avoid degradation of RNA. All experiments were conducted in an RNase free laboratory under a laminar flow cabinet. For long-term storage of RNA, the pure RNA was diluted 1 : 1 in a storage buffer (8 M guanidinium isothiocyanate, 80 mM Tris–HCl at pH 8.5, 24 mM MgCl2, 140 mM KCl, 0.4 Units ml−1 RNase Inhibitor) and stored at −80°C.
3. Results
3.1. Cell trapping and lysis
During cell preparation and re-suspension in appropriate buffers, the K. brevis vegetative motile cells formed temporary cysts owing to the mechanical shock of centrifugation and the difference in salinity between seawater (500 mM) and the iso-osmotic low-conductivity buffer (no salt present to reduce the electrical conductivity). Temporary cysts are non-motile cells without flagella, thus lacking the ability to swim, and form under adverse conditions [36]. Cysts have the same cell contents as vegetative cells [37] but their cell walls consist of cellulose, dinosporin and mucilage arranged in several layers [38]. Also, cysts are much more resistant to alkali and acid treatment [39] and are sufficiently robust to persist as fossils of palaeontological significance [40].
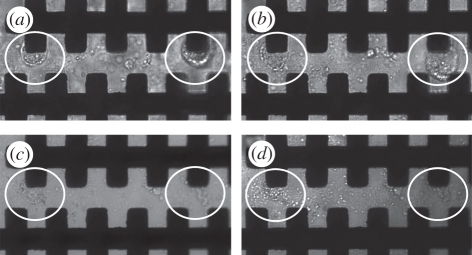
Temporary cysts were concentrated onto the electrodes using an AC voltage of 1 V and a frequency of 200 kHz, applied for 10 s. Figure 1a shows an image of the phytoplankton cells collecting at the electrode tips by positive DEP in iso-osmotic low-conductivity buffer. Positive DEP was observed for these cells over the range of measured frequencies from 70 to 600 kHz. Below 70 kHz, the cells either did not move or experienced slight negative DEP; frequencies higher than 600 kHz could not be measured because of the limited bandwidth of the amplifiers used in this work. These experimental observations are consistent with the DEP behaviour of viable cells [41–44]. When a cell is suspended in low-conductivity media (the conductivity of the buffer was 10.5 mS m−1), then over an intermediate frequency window of two to three decades, the polarizability of the cell is greater than the medium and positive DEP is observed. At high and low frequencies, the cell becomes less polarizable and experiences negative DEP. After collecting the cells, the frequency and voltage was changed to induce electroporation. Figure 2 shows that upon application of 60 V at 600 kHz, the cells deform and undergo morphological changes, as shown by the sequence of images. Two cells are shown (ringed), immediately after DEP trapping and then 15 s after application of a higher voltage. The figure shows that the cell membranes have been destroyed and the cytoplasm has leaked into the medium. Further confirmation of cell electroporation was obtained by examining the uptake of PI in figure 3. This molecule is weakly fluorescent in solution but its excitation maximum increases 20-fold when bound to DNA. It is membrane impermeant and is a good indicator of cell plasma membrane damage. Fluorescence images were acquired before and after electroporation at a frequency of 600 kHz and voltage of 45 V. This lower voltage was used to increase the time taken to achieve electroporation to 15 s because at 60 V electroporation was too fast to image. The PI was incorporated into the cells indicating that the membranes are electroporated.
Figure 1.
(a) Image of microchip using a UK pound coin for size reference. (b) Light microscopy image (20× magnification) of K. brevis cells captured on the micro-electrodes upon the application of 1 V at 200 kHz for 10 s.
Figure 2.
Membrane deformation and pore formation of K. brevis cells: (a) pre-electroporation under cell trapping of 1 V field at 200 kHz for 20 s. Two distinct cells, encircled white, can be observed trapped on the micro-electrodes. (b) Post-electroporation of 60 V field at 600 kHz for 5 s. Membranes of the encircled cells are becoming disrupted owing to the formation of pores by the continued application of an electric field. (c) Post-electroporation of 60 V at 600 kHz for 10 s. Poration of the encircled cells is so extensive that intracellular material is escaping into the iso-osmotic low-conductivity buffer. (d) Post-electroporation of 60 V at 600 kHz for 15 s, the encircled cells have become completely disrupted and no distinct cell membranes can be observed.
Figure 3.
Micrographs showing the process of single-cell electroporation of K. brevis temporary cysts. (a) Bright-field images on the application of 45 V at 600 kHz captured at (i) 0 s (ii) 30 s (iii) 1 min (iv) 1.5 min and (v) 2 min. (b) Epi-fluorescence images collected simultaneously at an excitation of 536 nm and emission of 593 nm.
Electroporation has been demonstrated for a wide variety of cell types including Escherichia coli [45], and occurs when the induced trans-membrane potential exceeds approximately 1 V [44,46,47]. The induced trans-membrane voltage is a function of the applied field magnitude and frequency. For the castellated electrodes used in this work, the electric field seen by the cell varies with position, but a typical value of electric field (at 1 V) is of the order 5 × 104 V m−1. The electrical potential that dropped across the cell membrane can be calculated provided the cell dielectric properties are known [48]. There is very little literature describing the dielectric properties of marine organisms, and we did not specifically measure the properties of K. brevis. However, we estimate the trans-membrane potential using values typically found for most cells. At an applied voltage of 60 V, the cell sees a field of the order 3 × 106 V m−1. For a cell radius of 10 µm (K. brevis), a suspending medium conductivity of 10 mS m−1, a frequency of 600 kHz and an assumed membrane capacitance of 8 mF m−2 (reported for similar marine alga [49,50]), the trans-membrane potential is calculated as 3 V, more than sufficient to cause dielectric breakdown of the membrane and complete cell lysis [44,46]. The experimental conditions used for cell lysis were selected to maximize the applied voltage dropped across the cell membrane. If the frequency is too high, the field is no longer dropped across the membrane, while at low frequencies the electrical potential in the suspending medium is reduced owing to electrode polarization. For this reason, a frequency in the region of 600 kHz was used.
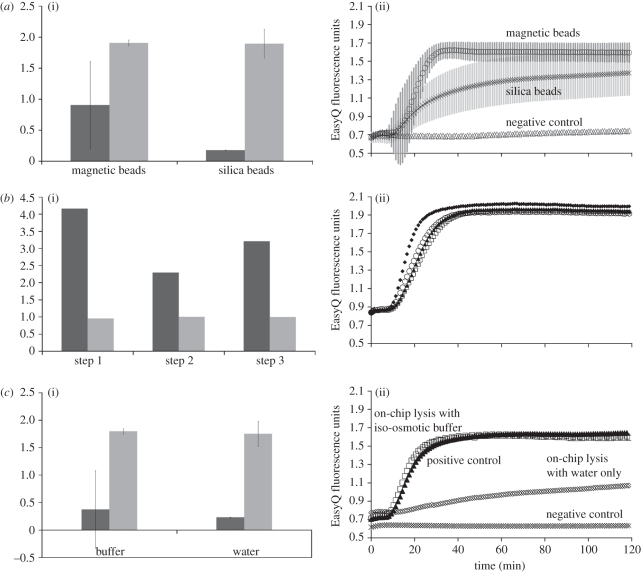
NASBA analysis was used to quantify the lysis efficiency by measuring the amount and purity of extracted RNA. Two different RNA extraction and purification methods were compared, using lysate from electroporated cells. The methods were based on extraction using (i) magnetic beads based on the Boom technology [51], and (ii) silica beads. The latter is the most commonly used method of RNA extraction and purification, and uses silica beads in packed columns. Magnetic beads are an attractive alternative for miniaturization because they can be easily manipulated within microfluidic systems and have been used with an LOC for DNA extraction [52]. Figure 4a compares these two methods in terms of purity and yield (measured spectrophotometrically) together with the NASBA fluorescence data. It shows that both methods gave RNA of comparable purity; however, the magnetic bead method yielded approximately five times more RNA. The NASBA data show that the RNA is also of better quality because it amplifies more efficiently, as demonstrated by the initial gradient of the curve.
Figure 4.
Assessment of bench-top RNA extraction and purification methods. These data were obtained from 250 µl containing 80 000 cells, electroporated on-chip with a 30 V field at 600 kHz for 20 s: (a) comparison of extraction using magnetic and silica beads in a low-conductivity iso-osmotic buffer: (i) yield (black bars, ng µl−1) and purity (grey bars, AU), and (ii) NABSA amplification data. Error bars are the standard deviation of a triplicate experiment. The magnetic beads extraction and purification method produced RNA of comparable relative purity to the silica columns as seen in (i). However, both the yield and NASBA amplification efficiency were far better for the magnetic beads. (b) Effect of three subsequent centrifugation steps before loading the sample on the electroporation chip in low-conductivity iso-osmotic buffer: (i) yield (black bars) and relative purity (grey bars), and (ii) NABSA amplification data. Black diamonds, positive control; white circles, one centrifugation; black triangles, two centrifugations; white squares, three centrifugations. Yield and purity data are normalized to the commercial lysis buffer. The centrifugation steps appear to compromise the NASBA amplification efficiency of the extracted RNA, as seen in (ii), while yield appears to fluctuate counterintuitively with increasing centrifugation steps. (c) Effect of low-conductivity iso-osmotic buffer: (i) yield (black bars) and purity (grey bars), and (ii) NABSA amplification data. Yield and purity data are normalized to the commercial lysis buffer. Electroporation in both the low-conductivity iso-osmotic buffer and in water yielded similar quantities of RNA but more than using a commercial lysis buffer. Both had lower spectrophotometric purity. NASBA data showed RNA degradation in water resulting in a lower initial gradient.
In order to evaluate the effect of sample pre-processing on the RNA yield, the NASBA amplification of cell pellets was performed after centrifugation, sample agitation and re-suspension, but without electroporation. The RNA was purified by magnetic beads and amplified with NASBA. The results shown in figure 4b are for samples after one, two and three centrifugation/wash steps, for three separate experiments in iso-osmotic low-conductivity buffer. The yield and purity data are normalized to the result using a commercial RNA lysis buffer. This was used to extract RNA from the same culture and the same number of cells on the same day, i.e. a positive control. The figure shows that centrifugation/wash steps partially lyse the cells, releasing RNA into the buffer, which can be amplified by NASBA. The amount of RNA is between two and four times greater than that released using commercial lysis buffer (positive control), and the relative purity is similar (figure 4b).
Cells were also re-suspended in a low-osmolarity medium (water) and then electroporated. The results are shown in figure 4c, and demonstrate that the combination of hypotonic lysis and electroporation yielded a similar amount of RNA to electroporated cells in a low-osmolarity buffer. Electroporation in both low-osmolarity and hypertonic buffers yielded more RNA than using the commercial lysis buffer only. However, the NASBA amplification of the hypotonic electroporation product has lower initial gradient indicating that the RNA was probably highly fragmented when suspended in water.
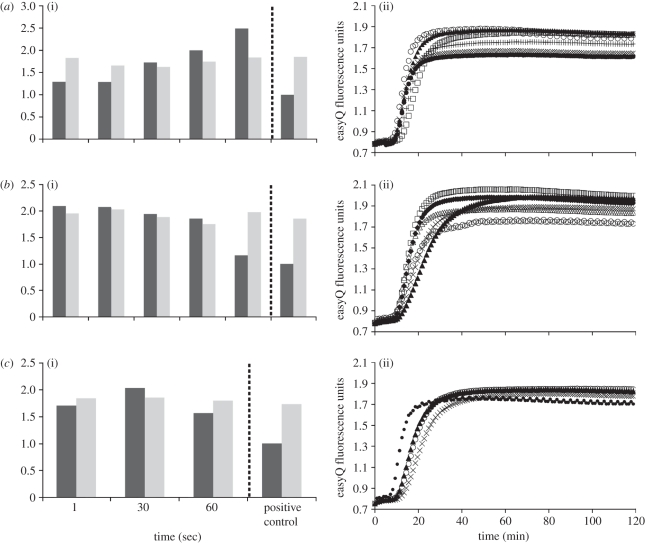
Quantitative experiments were performed to establish the optimal electroporation conditions suitable for on-chip lysis and RNA extraction. The yield and purity of RNA (extracted by magnetic beads) was evaluated for different applied voltages and times. Data summarized in figure 5 show it to be a function of time for three different voltages: 1, 30 and 60 V. The yield and purity data are expressed normalized to control experiments with RNA extracted using the commercial lysis buffer (for the same cell concentration). A negative control experiment indicated that some RNA was always present in the sample, but the amount was small and highly variable. The lowest potential that reduced this variability was 1 V and is therefore the lowest potential for which we report results. Figure 5a shows data for electric field-mediated cell lysis at 1 V and indicates that RNA yield increases with time of exposure. Increasing the voltage to 30 V or 60 V gives the data in figure 5b,c. The RNA yield is high and comparable to that obtained after 120 s at 1 V (figure 5a). However, for the 30 V data, the yield is approximately twice that obtained using the commercial buffer but diminishes with time. At 60 V, yield is also better than the commercial buffer but the RNA degrades with time. Electroporation for longer than 60 s caused boiling at the electrodes and loss of the sample.
Figure 5.
Identification of optimal electroporation conditions: (a) On-chip lysis using a 1 V field for five different time intervals: (i) yield and purity results (black bars, relative yield; grey bars, relative fluorescence) and (ii) NABSA amplification data. Triangles, positive control; white circles, 1 s; crosses, 30 s; black circles, 60 s; plus symbols, 90 s; squares, 120 s. Increasing duration of electroporation led to increased yield of RNA extracted from the cell lysate. (b) On-chip lysis using a 30 V field for five different time intervals: (i) Yield and purity results (black bars, relative yield; grey bars, relative fluorescence) and (ii) NABSA amplification data. White triangles, positive control; diamonds, 1 s; squares, 30 s; crosses, 60 s; circles, 90 s; black triangles, 120 s. Electroporation that lasted longer than 60 s caused the yield of RNA extracted from the cell lysate to decrease. (c) On-chip lysis using a 60 V field for different time intervals: (i) Yield and purity results (black-filled bars, relative yield; grey-filled bars, relative fluorescence) and (ii) NABSA amplification data. Black circles, positive control; triangles, 1 s; white circles, 30 s; crosses, 60 s. Data show that electroporation of 60 V was more effective than the bench-top alternative but appeared to degrade the quality of extracted RNA from the lysate.
4. Conclusions and future directions
This work has demonstrated dielectrophoretic concentration and electric field-mediated cell lysis of the marine microalga K. brevis. We used temporary cysts, which are non-mammalian targets chosen for their high resistance and the fact that they are difficult to break. For the same reasons, other laboratories have used bacterial spores of Bacillus subtilis and Bacillus thuringiensis, non-pathogenic simulants of anthrax [53,54] and Bacillus subtilis var. Bacillus niger [55].
Electric field-mediated cell lysis produced a high yield of RNA and in most cases pure RNA with amplification efficiency that was higher than the commercial lysis buffer. High voltages did not interfere with the amplification and detection of the RNA target, but yield was diminished after long-term exposure to the field. In terms of developing a system for sensitive and accurate RNA extraction and amplification, the optimum conditions are either a long exposure of cells to a low voltage (120 s, 1 V) or short exposure at higher voltage (1 s, 30 V), both of which give the best quality and quantity of RNA. Cell trapping and capture efficiency was estimated at nearly 100 per cent (ignoring cell losses during experimental manipulation).
The amount of total RNA extracted from each cell using electric field-mediated cell lysis was around 15 pg, well within the expected range of 10–30 pg for typical cells [56]. An assay sensitivity of one cell (or 0.1 fg of RNA) has been reported using bench-top lysis, extraction and amplification methods [35] showing that NASBA can detect and amplify as little as 30 RNA molecules [57].
Our method has the potential for integration within a complete microfluidic system for sub-cellular analysis of RNA using NASBA. A typical LOC system would include RNA extraction and purification, and real-time quantitative species-specific nucleic acid detection. RNA quantification would be achieved using a synthetic sequence of internal control RNA of known concentration, as used in an existing hand-held NASBA system [4].
Acknowledgements
M.N.T. developed the experimental protocols and cultured the microalgae. M.M.B. performed all on-chip lyses and nanodrop spectrophotometric quantification. M.N.T. extracted and purified the RNA and carried out all NASBA assays. M.M.B. acquired microscopy images. M.N.T. and M.M.B. analysed the data. M.C.M., M.N.T. and H.M. supervised the work and wrote the paper. M.C.M. and H.M. secured funding. The authors would like to acknowledge funding support by EU FP7 LABONFOIL grant project 224306 and a NOCS/NERC studentship for M.M.B. Also thanks to Nicolas Green for the electroporation chip, to Layla Hazeem of the Purdie laboratory for the initial culture of K. brevis and to Bethan Jones for help with the identification of temporary cysts.
References
- 1.Granéli E., Turner T. J. (eds) 2008. Ecology of harmful algae. Ecological studies. New York, NY: Springer [Google Scholar]
- 2.Prien R. D. 2007. The future of chemical in situ sensors. Mar. Chem. 107, 422–432 10.1016/j.marchem.2007.01.014 (doi:10.1016/j.marchem.2007.01.014) [DOI] [Google Scholar]
- 3.Moore T. S., Mullaugh K. M., Holyoke R. R., Madison A. S., Ycel M., Luther G. W. 2009. Marine chemical technology and sensors for marine waters: potentials and limits. Ann. Rev. Mar. Sci. 1, 91–115 10.1146/annurev.marine.010908.163817 (doi:10.1146/annurev.marine.010908.163817) [DOI] [PubMed] [Google Scholar]
- 4.Casper E. T., Patterson S. S., Bhanushali P., Farmer A., Smith M., Fries D. P., Paul J. H. 2007. A handheld NASBA analyzer for the field detection and quantification of Karenia brevis. Harmful Algae 6, 112–118 10.1016/j.hal.2006.11.001 (doi:10.1016/j.hal.2006.11.001) [DOI] [Google Scholar]
- 5.Ulrich R. M., Casper E. T., Campbell L., Richardson B., Heil C. A., Paul J. H. 2010. Detection and quantification of Karenia mikimotoi using real-time nucleic acid sequence-based amplification with internal control RNA (IC-NASBA). Harmful Algae 9, 116–122 10.1016/j.hal.2009.08.010 (doi:10.1016/j.hal.2009.08.010) [DOI] [Google Scholar]
- 6.Compton J. 1991. Nucleic acid sequence-based amplification. Nature 350, 91–92 10.1038/350091a0 (doi:10.1038/350091a0) [DOI] [PubMed] [Google Scholar]
- 7.Price C. W., Leslie D. C., Landers J. P. 2009. Nucleic acid extraction techniques and application to the microchip. Lab. Chip 9, 2484–2494 10.1039/b907652m (doi:10.1039/b907652m) [DOI] [PubMed] [Google Scholar]
- 8.Wen J., Legendre L. A., Bienvenue J. M., Landers J. P. 2008. Purification of nucleic acids in microfluidic devices. Anal. Chem. 80, 6472–6479 10.1021/ac8014998 (doi:10.1021/ac8014998) [DOI] [PubMed] [Google Scholar]
- 9.Horsman K. M., Bienvenue J. M., Blasier K. R., Landers J. P. 2007. Forensic DNA analysis on microfluidic devices: a review. J. Forensic Sci. 52, 784–799 10.1111/j.1556-4029.2007.00468.x (doi:10.1111/j.1556-4029.2007.00468.x) [DOI] [PubMed] [Google Scholar]
- 10.Chao T.-C., Ros A. 2008. Microfluidic single-cell analysis of intracellular compounds. J. R. Soc. Interface 5, S139–S150 10.1098/rsif.2008.0233.focus (doi:10.1098/rsif.2008.0233.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lui C., Cady N. C., Batt C. A. 2009. Nucleic acid-based detection of bacterial pathogens using integrated microfluidic platform systems. Sensors 9, 3713–3744 10.3390/s90503713 (doi:10.3390/s90503713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ligler F. S. 2009. Perspective on optical biosensors and integrated sensor systems. Anal. Chem. 81, 519–526 10.1021/ac8016289 (doi:10.1021/ac8016289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharyya A., Klapperich C. M. 2008. On-chip cell lysis. In Encyclopedia of microfluidics and nanofluidics, vol. 3 (ed. Li D.), pp. 1513–1515 Berlin, Germany: Springer [Google Scholar]
- 14.Di Carlo D., Jeong K.-H., Lee L. P. 2003. Reagentless mechanical cell lysis by nanoscale barbs in microchannels for sample preparation. Lab. Chip 3, 287–291 10.1039/b305162e (doi:10.1039/b305162e) [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J. F., Russell D. W. (eds) 2000. Molecular cloning: a laboratory manual. New York, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- 16.Wang H.-Y., Bhunia A. K., Lu C. 2006. A microfluidic flow-through device for high throughput electrical lysis of bacterial cells based on continuous DC voltage. Biosens. Bioelectron. 22, 582–588 10.1016/j.bios.2006.01.032 (doi:10.1016/j.bios.2006.01.032) [DOI] [PubMed] [Google Scholar]
- 17.Lee H. J., Kim J.-H., Lim H. K., Cho E. C., Huh N., Ko C., Park J. C., Choi J.-W., Lee S. S. 2010. Electrochemical cell lysis device for DNA extraction. Lab. Chip 10, 626–633 10.1039/b916606h (doi:10.1039/b916606h) [DOI] [PubMed] [Google Scholar]
- 18.Liu R. H., Yang J., Lenigk R., Bonanno J., Grodzinski P. 2004. Self-contained, fully integrated biochip for sample preparation, polymerase chain reaction amplification, and DNA microarray detection. Anal. Chem. 76, 1824–1831 10.1021/ac0353029 (doi:10.1021/ac0353029) [DOI] [PubMed] [Google Scholar]
- 19.Kim J., Johnson M., Hill P., Gale B. K. 2009. Microfluidic sample preparation: cell lysis and nucleic acid purification. Integr. Biol. 1, 574–586 10.1039/b905844c (doi:10.1039/b905844c) [DOI] [PubMed] [Google Scholar]
- 20.Sims C. E., Allbritton N. L. 2007. Analysis of single mammalian cells on-chip. Lab. Chip 7, 423–440 10.1039/b615235j (doi:10.1039/b615235j) [DOI] [PubMed] [Google Scholar]
- 21.Brown R. B., Audet J. 2008. Current techniques for single-cell lysis. J. R. Soc. Interface 5, S131–S138 10.1098/rsif.2008.0009.focus (doi:10.1098/rsif.2008.0009.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y., Mather E. L., Bell J. L., Madou M. 2002. MEMS-based sample preparation for molecular diagnostics. Anal. Bioanal. Chem. 372, 49–65 10.1007/s00216-001-1191-9 (doi:10.1007/s00216-001-1191-9) [DOI] [PubMed] [Google Scholar]
- 23.Lagally E., Soh H. 2005. Integrated genetic analysis microsystems. Crit. Rev. Solid State Mater. Sci. 30, 207–233 10.1080/10408430500332149 (doi:10.1080/10408430500332149) [DOI] [Google Scholar]
- 24.Lapizco-Encinas B. H., Rito-Palomares M. 2007. Dielectrophoresis for the manipulation of nanobioparticles. Electrophoresis 28, 4521–4538 10.1002/elps.200700303 (doi:10.1002/elps.200700303) [DOI] [PubMed] [Google Scholar]
- 25.Archer S., Li T.-T., Evans A. T., Britland S. T., Morgan H. 1999. Cell reactions to dielectrophoretic manipulation. Biochem. Biophys. Res. Comm. 257, 687–698 10.1006/bbrc.1999.0445 (doi:10.1006/bbrc.1999.0445) [DOI] [PubMed] [Google Scholar]
- 26.Coster H. G. 1965. A quantitative analysis of the voltage-current relationships of fixed charge membranes and the associated property of ‘punch-through’. Biophys. J. 5, 669–686 10.1016/S0006-3495(65)86745-5 (doi:10.1016/S0006-3495(65)86745-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weaver J. C., Chizmadzhev Y. A. 1996. Theory of electroporation: a review. Bioelectrochem. Bioenerg. 41, 135–160 10.1016/S0302-4598(96)05062-3 (doi:10.1016/S0302-4598(96)05062-3) [DOI] [Google Scholar]
- 28.Lee S.-W., Tai Y.-C. 1999. A micro cell lysis device. Sens. Actuators A Phys. 73, 74–79 10.1016/S0924-4247(98)00257-X (doi:10.1016/S0924-4247(98)00257-X) [DOI] [Google Scholar]
- 29.Lin Y.-C., Jen C.-M., Huang M.-Y., Wu C.-Y., Lin X.-Z. 2001. Electroporation microchips for continuous gene transfection. Sens. Actuators B Chem. 79, 137–143 10.1016/S0925-4005(01)00859-0 (doi:10.1016/S0925-4005(01)00859-0) [DOI] [Google Scholar]
- 30.Huang Y., Rubinsky B. 2003. Flow-through micro-electroporation chip for high efficiency single-cell genetic manipulation. Sens. Actuators A Phys. 104, 205–212 10.1016/S0924-4247(03)00050-5 (doi:10.1016/S0924-4247(03)00050-5) [DOI] [Google Scholar]
- 31.Lu H., Schmidt M. A., Jensen K. F. 2005. A microfluidic electroporation device for cell lysis. Lab. Chip 5, 23–29 10.1039/b406205a (doi:10.1039/b406205a) [DOI] [PubMed] [Google Scholar]
- 32.Ikeda N., Tanaka N., Yanagida Y., Hatsuzawa T. 2007. On-chip single-cell lysis for extracting intracellular material. Jpn J. Appl. Phys. 46, 6410–6414 10.1143/JJAP.46.6410 (doi:10.1143/JJAP.46.6410) [DOI] [Google Scholar]
- 33.de la Rosa C., Tilley P. A., Fox J. D., Kaler K. V. I. 2008. Microfluidic device for dielectrophoresis, manipulation and electrodisruption of respiratory pathogen Bordetella pertussis. Biomed. Eng. IEEE Trans. 55, 2426–2432 10.1109/TBME.2008.923148 (doi:10.1109/TBME.2008.923148) [DOI] [PubMed] [Google Scholar]
- 34.Sedgwick H., Caron F., Monaghan P. B., Kolch W., Cooper J. M. 2008. Lab-on-a-chip technologies for proteomic analysis from isolated cells. J. R. Soc. Interface 5, S123–S130 10.1098/rsif.2008.0169.focus (doi:10.1098/rsif.2008.0169.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casper E. T., Paul J. H., Smith M. C., Gray M. 2004. Detection and quantification of the red tide dinoflagellate Karenia brevis by real-time nucleic acid sequence-based amplification. Appl. Environ. Microbiol. 70, 4727–4732 10.1128/aem.70.8.4727-4732.2004 (doi:10.1128/aem.70.8.4727-4732.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson D. M., Fukuyo Y., Matsuoka K. 2003. Cyst methodologies. In Manual on harmful marine microalgae (eds Hallegraeff G. M., Anderson D. M., Cembella A. D.), pp. 166–170 Paris, France: UNESCO Publishing [Google Scholar]
- 37.Anderson D. M. 1980. Effects of temperature conditioning on development and germination of Gonyaulax tamarensis (Dinophyceae) hypnozygotes. J. Phycol. 16, 166–172 10.1111/j.1529-8817.1980.tb03013.x (doi:10.1111/j.1529-8817.1980.tb03013.x) [DOI] [Google Scholar]
- 38.Graham L. E., Graham J. M., Wilcox L. W. 2009. Algae. San Fransisco, CA: Pearson [Google Scholar]
- 39.Bold H. C., Wynne M. J. 1978. Introduction to algae: structure and reproduction. Upper Sadle River, NJ: Prentice-Hall Inc [Google Scholar]
- 40.Dale B. 2001. The sedimentary record of dinoflagellate cysts: looking back into the future of phytoplankton blooms. Scientia Marina 65(Suppl. 2), 254–272 10.3989/scimar.2001.65s2257 (doi:10.3989/scimar.2001.65s2257) [DOI] [Google Scholar]
- 41.Jones T. B. 1995. Electromechanics of particles. Cambridge, UK: Cambridge University Press [Google Scholar]
- 42.Morgan H., Green N. G. 2003. AC electrokinetics: colloids and nanoparticles. Baldock, UK: Research Studies Press Ltd [Google Scholar]
- 43.Voldman J. 2006. Electrical forces for microscale cell manipulation. Annu. Rev. Biomed. Eng. 8, 425–454 10.1146/annurev.bioeng.8.061505.095739 (doi:10.1146/annurev.bioeng.8.061505.095739) [DOI] [PubMed] [Google Scholar]
- 44.Pethig R., Markx G. H. 1997. Applications of dielectrophoresis in biotechnology. Trends Biotechnol. 15, 426–432 10.1016/s0167-7799(97)01096-2 (doi:10.1016/s0167-7799(97)01096-2) [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann U., Schulz J., Pilwat G. 1973. Transcellular ion flow in Escherichia coli B and electrical sizing of bacterias. Biophys. J. 13, 1005–1013 10.1016/S0006-3495(73)86041-2 (doi:10.1016/S0006-3495(73)86041-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver J. C. 1993. Electroporation: a general phenomenon for manipulating cells and tissues. J. Cell. Biochem. 51, 426–435 [DOI] [PubMed] [Google Scholar]
- 47.Pethig R., Jakubek L. M., Sanger R. H., Heart E., Corson E. D., Smith P. J. S. 2005. Electrokinetic measurements of membrane capacitance and conductance for pancreatic beta cells. Nanobiotechnol. IEE Proc. 152, 189–193 10.1049/ip-nbt:20050040 (doi:10.1049/ip-nbt:20050040) [DOI] [PubMed] [Google Scholar]
- 48.Grosse C., Schwan H. P. 1992. Cellular membrane potentials induced by alternating fields. Biophys. J. 63, 1632–1642 10.1016/S0006-3495(92)81740-X (doi:10.1016/S0006-3495(92)81740-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Müller T., Schnelle T., Fuhr G. 1998. Dielectric single cell spectra in snow algae. Polar Biol. 20, 303–310 10.1007/s003000050307 (doi:10.1007/s003000050307) [DOI] [Google Scholar]
- 50.Wang J., Sukhorukov V. L., Djuzenova C. S., Zimmermann U., Müller T., Fuhr G. 1997. Electrorotational spectra of protoplasts generated from the giant marine alga Valonia utricularis. Protoplasma 196, 123–134 10.1007/BF01279561 (doi:10.1007/BF01279561) [DOI] [Google Scholar]
- 51.Boom R., Sol C. J. A., Salimans M. M. M., Jansen C. L., Wertheim-Van Dillen P. M. E., Van der Noordaa J. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28, 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lien K.-Y., Liu C.-J., Lin Y.-C., Kuo P.-L., Lee G.-B. 2009. Extraction of genomic DNA and detection of single nucleotide polymorphism genotyping utilizing an integrated magnetic bead-based microfluidic platform. Microfluid. Nanofluid. 6, 539–555 10.1007/s10404-008-0337-x (doi:10.1007/s10404-008-0337-x) [DOI] [Google Scholar]
- 53.Belgrader P., Young S., Yuan B., Primeau M., Christel L. A., Pourahmadi F., Northrup M. A. 2000. A battery-powered notebook thermal cycler for rapid multiplex real-time PCR analysis. Anal. Chem. 73, 286–289 10.1021/ac000905v (doi:10.1021/ac000905v) [DOI] [PubMed] [Google Scholar]
- 54.Lapizco-Encinas B. H., Davalos R. V., Simmons B. A., Cummings E. B., Fintschenko Y. 2005. An insulator-based (electrodeless) dielectrophoretic concentrator for microbes in water. J. Microbiol. Meth. 62, 317–326 10.1016/j.mimet.2005.04.027 (doi:10.1016/j.mimet.2005.04.027) [DOI] [PubMed] [Google Scholar]
- 55.Hoettges K. F., Hughes M. P., Cotton A., Hopkins N. A. E., McDonnell M. B. 2003. Optimizing particle collection for enhanced surface-based biosensors. Eng. Med. Biol. Mag. IEEE 22, 68–74 10.1109/MEMB.2003.1266049 (doi:10.1109/MEMB.2003.1266049) [DOI] [PubMed] [Google Scholar]
- 56.Alberts B., Johnson A., Walter P., Lewis J., Raff M., Roberts K. 2008. Molecular cell biology. New York, NY: Garland Publishing Inc [Google Scholar]
- 57.Sooknanan R., Van Gemen B., Malek L. 1995. Nucleic acid sequence-based amplification. In Molecular methods for virus detection (eds Wiedbrauk D. L., Farkas D. H.), pp. 261–285 New York, NY: Academic Press [Google Scholar]