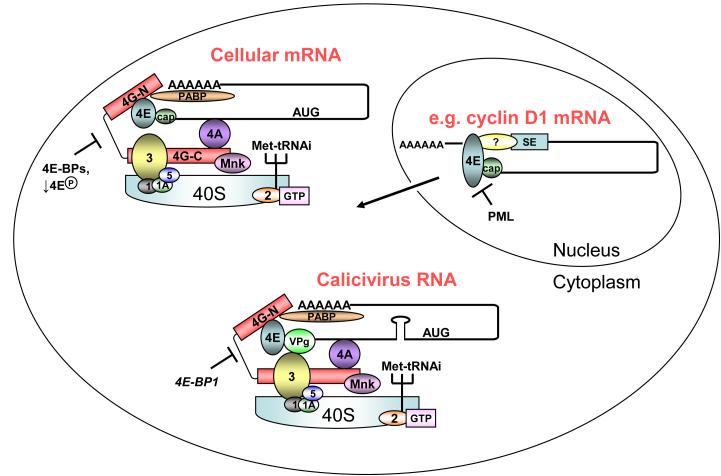
Figure 2. The different functions of eIF4E.
The long-established role of eIF4E in the cytoplasm is in the initiation of cap-dependent translation on cellular mRNAs. eIF4E is the cap-binding protein component of the eIF4F complex, which includes the RNA helicase eIF4A and the scaffolding protein eIF4G. Binding of eIF4E to the cap structure on the 5′ end of cellular mRNAs recruits the eIF4F complex to the mRNA and in turn, the 40S ribosomal subunit. Binding of eIF4E to eIF4G is in competition with the 4E-binding proteins (4E-BPs) and binding to the cap is regulated by the phosphorylation status of eIF4E (4E-P). A role for eIF4E in calicivirus RNA translation has recently been discovered (see text). Here, eIF4E binds to the calicivirus VPg protein attached to the 5′ end of the viral RNA; this interaction is required for translation initiation on some, but not all, calicivirus RNAs. The interaction between eIF4E and eIF4G is inhibited by 4E-BP1 and this results in inhibition of the translation of feline calicivirus RNA but not murine norovirus RNA (hence, the italics in the figure). Binding of eIF3 to norovirus VPg has also been reported, although no functional role for this interaction has yet been defined. Roles for eIF4A (known to be required for calicivirus translation), PABP and other factors are currently being investigated. In the nucleus, eIF4E is known to interact with the 5′ cap structure and function in the export of specific mRNAs (e.g. cyclin D1) from the nucleus to the cytoplasm. eIF4E recognises a “specificity element” within the 3′ UTR of the mRNA, possibly through interaction with an unknown factor (designated ?), that defines which mRNAs are exported. Binding of proteins such as PML to eIF4E inhibits the eIF4E-cap interaction and, therefore, the export of mRNAs.